Phylogenetic Relationships in the Cactus Family (Cactaceae) Based on Evidence from Trnk/Matk and Trnl-Trnf Sequences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Haseltonia Articles and Authors.Xlsx
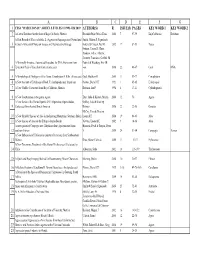
ABCDEFG 1 CSSA "HASELTONIA" ARTICLE TITLES #1 1993–#26 2019 AUTHOR(S) R ISSUE(S) PAGES KEY WORD 1 KEY WORD 2 2 A Cactus Database for the State of Baja California, Mexico Resendiz Ruiz, María Elena 2000 7 97-99 BajaCalifornia Database A First Record of Yucca aloifolia L. (Agavaceae/Asparagaceae) Naturalized Smith, Gideon F, Figueiredo, 3 in South Africa with Notes on its uses and Reproductive Biology Estrela & Crouch, Neil R 2012 17 87-93 Yucca Fotinos, Tonya D, Clase, Teodoro, Veloz, Alberto, Jimenez, Francisco, Griffith, M A Minimally Invasive, Automated Procedure for DNA Extraction from Patrick & Wettberg, Eric JB 4 Epidermal Peels of Succulent Cacti (Cactaceae) von 2016 22 46-47 Cacti DNA 5 A Morphological Phylogeny of the Genus Conophytum N.E.Br. (Aizoaceae) Opel, Matthew R 2005 11 53-77 Conophytum 6 A New Account of Echidnopsis Hook. F. (Asclepiadaceae: Stapeliae) Plowes, Darrel CH 1993 1 65-85 Echidnopsis 7 A New Cholla (Cactaceae) from Baja California, Mexico Rebman, Jon P 1998 6 17-21 Cylindropuntia 8 A New Combination in the genus Agave Etter, Julia & Kristen, Martin 2006 12 70 Agave A New Series of the Genus Opuntia Mill. (Opuntieae, Opuntioideae, Oakley, Luis & Kiesling, 9 Cactaceae) from Austral South America Roberto 2016 22 22-30 Opuntia McCoy, Tom & Newton, 10 A New Shrubby Species of Aloe in the Imatong Mountains, Southern Sudan Leonard E 2014 19 64-65 Aloe 11 A New Species of Aloe on the Ethiopia-Sudan Border Newton, Leonard E 2002 9 14-16 Aloe A new species of Ceropegia sect. -

02.2020 Central Spine Final
FEBRUARY 2020 Copiapoa longistaminea Photo credit: Woody Minnich IMPORTANT FEBRUARY DATE CHANGES Cliff and Tammy Fielding Open Garden: Saturday February 15, 2020 Monthly meeting, Woody Minnich: Sunday February 16, 2020 Be sure to visit CACSS on the web at: centralarizonacactus.org, Facebook, Instagram and YouTube at: CentralArizonaCactus, and members only at: CentralArizonaCactus Swap and Shop. CACSS 1 February 2020 20 YEARS IN THE ATACAMA, LAND OF THE COPIAPOAS Photos and Text by Wendell S. ‘Woody’ Minnich Similar to the coast of Namibia, the coastal and inland regions of northern Chile, known as the Atacama, are mainly watered by amazing fogs, “the Camanchacas.” These fog- fed regions, in two of the driest deserts in the world, have some of the most interesting cactus and succulents to be found anywhere. The Atacama of northern Chile has an endemic genus considered by many to be one of the most dramatic to have ever evolved-the Copiapoa. This ancient genus is also believed to be tens of thousands of years old, and there are those who feel it might well be on its way out! The ocean currents that affect the coastal Atacama have changed considerably over the last hundreds of years, and now its only source of moisture is primarily from consistent dense fogs. Some of these areas rarely, if ever, get rain, and the plants that have evolved there live almost entirely off the heavy condensation from the Camanchaca. There are many different Copiapoa species ranging from small quarter-sized subterranean geophytes, to giant 1,000-year-old, 300-head mounding clusters. -

Floristic and Ecological Characterization of Habitat Types on an Inselberg in Minas Gerais, Southeastern Brazil
Acta Botanica Brasilica - 31(2): 199-211. April-June 2017. doi: 10.1590/0102-33062016abb0409 Floristic and ecological characterization of habitat types on an inselberg in Minas Gerais, southeastern Brazil Luiza F. A. de Paula1*, Nara F. O. Mota2, Pedro L. Viana2 and João R. Stehmann3 Received: November 21, 2016 Accepted: March 2, 2017 . ABSTRACT Inselbergs are granitic or gneissic rock outcrops, distributed mainly in tropical and subtropical regions. Th ey are considered terrestrial islands because of their strong spatial and ecological isolation, thus harboring a set of distinct plant communities that diff er from the surrounding matrix. In Brazil, inselbergs scattered in the Atlantic Forest contain unusually high levels of plant species richness and endemism. Th is study aimed to inventory species of vascular plants and to describe the main habitat types found on an inselberg located in the state of Minas Gerais, in southeastern Brazil. A total of 89 species of vascular plants were recorded (belonging to 37 families), of which six were new to science. Th e richest family was Bromeliaceae (10 spp.), followed by Cyperaceae (seven spp.), Orchidaceae and Poaceae (six spp. each). Life forms were distributed in diff erent proportions between habitats, which suggested distinct microenvironments on the inselberg. In general, habitats under similar environmental stress shared common species and life-form proportions. We argue that fl oristic inventories are still necessary for the development of conservation strategies and management of the unique vegetation on inselbergs in Brazil. Keywords: endemism, granitic and gneissic rock outcrops, life forms, terrestrial islands, vascular plants occurring on rock outcrops within the Atlantic Forest Introduction domain, 416 are endemic to these formations (Stehmann et al. -

Copyright Notice
Copyright Notice This electronic reprint is provided by the author(s) to be consulted by fellow scientists. It is not to be used for any purpose other than private study, scholarship, or research. Further reproduction or distribution of this reprint is restricted by copyright laws. If in doubt about fair use of reprints for research purposes, the user should review the copyright notice contained in the original journal from which this electronic reprint was made. Journal of Vegetation Science 7: 667-680, 1996 © IAVS; Opulus Press Uppsala. Printed in Sweden - Biogeographic patterns of Argentine cacti - 667 Species richness of Argentine cacti: A test of biogeographic hypotheses Mourelle, Cristina & Ezcurra, Exequiel Centro de Ecología, UNAM, Apartado Postal 70-275, 04510 - Mexico, D.F., Mexico; Fax +52 5 6228995; E-mail [email protected] Abstract. Patterns of species richness are described for 50 cacti and (e) pereskioid cacti. Columnar species have columnar, 109 globose and 50 opuntioid cacti species in 318 column-like stems with ribs, formed by an arrangement grid cells (1°×1°) covering Argentina. Biological richness of the areoles in longitudinal rows. These species have hypotheses were tested by regressing 15 environmental parallel vascular bundles, separated by succulent paren- descriptors against species richness in each group. We also chyma, sometimes fusing towards a woody base in the included the collection effort (estimated as the logarithm of the number of herbarium specimens collected in each cell) to adults. We broadly considered as columnar cacti: estimate the possible error induced by underrepresentation in candelabriform arborescent species, unbranched erect certain cells. -

Reproductive Biology, Hybridization, and Flower Visitors of Rare Sclerocactus Taxa in Utah's Uintah Basin
Western North American Naturalist Volume 70 Number 3 Article 10 10-11-2010 Reproductive biology, hybridization, and flower visitors of rare Sclerocactus taxa in Utah's Uintah Basin Vincent J. Tepedino Utah State University, Logan, [email protected] Terry L. Griswold Utah State University, Logan, [email protected] William R. Bowlin Utah State University, Logan Follow this and additional works at: https://scholarsarchive.byu.edu/wnan Recommended Citation Tepedino, Vincent J.; Griswold, Terry L.; and Bowlin, William R. (2010) "Reproductive biology, hybridization, and flower visitors of rare Sclerocactus taxa in Utah's Uintah Basin," Western North American Naturalist: Vol. 70 : No. 3 , Article 10. Available at: https://scholarsarchive.byu.edu/wnan/vol70/iss3/10 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Western North American Naturalist 70(3), © 2010, pp. 377–386 REPRODUCTIVE BIOLOGY, HYBRIDIZATION, AND FLOWER VISITORS OF RARE SCLEROCACTUS TAXA IN UTAH’S UINTAH BASIN Vincent J. Tepedino1,2, Terry L. Griswold1, and William R. Bowlin1,3 ABSTRACT.—We studied the mating system and flower visitors of 2 threatened species of Sclerocactus (Cactaceae) in the Uintah Basin of eastern Utah—an area undergoing rapid energy development. We found that both S. wetlandicus and S. brevispinus are predominantly outcrossed and are essentially self-incompatible. A third presumptive taxon (unde- scribed; here called S. wetlandicus-var1) is fully self-compatible but cannot produce seeds unless the flowers are visited by pollinators. -

Elaboración De Una Guía Ilustrada De Cactáceas En Honduras
Elaboración de una guía ilustrada de Cactáceas en Honduras Juan Pablo Schulze Rojas ZAMORANO Carrera de Desarrollo Socioeconómico y Ambiente Diciembre, 2004 i Elaboración de una guía ilustrada de Cactáceas en Honduras Proyecto especial presentado como requisito parcial para optar al título de Ingeniero en Desarrollo Socioeconómico y Ambiente en el Grado Académico de Licenciatura. Presentado por: Juan Pablo Schulze Rojas Honduras Diciembre, 2004 ii El autor concede a Zamorano permiso para reproducir y distribuir copias de este trabajo para fines educativos. Para otras personas físicas o jurídicas se reservan los derechos de autor. ________________________________ Juan Pablo Schulze Rojas Honduras Diciembre, 2004 iii Elaboración de una guía ilustrada de Cactáceas en Honduras Presentado por Juan Pablo Schulze Rojas Aprobada: __________________________ __________________________ José L. Linares, Ing. Agr. Mayra Falck, M.Sc. Asesor Principal Coordinadora de la Carrera de Desarrollo Socioeconómico y Ambiente __________________________ __________________________ George Pilz, Ph.D. Aurelio Revilla, M.S.A. Asesor Decano Académico Interino __________________________ Kenneth L. Hoadley, D.B.A. Rector iv DEDICATORIA A mi mamá Toya. A mi papá Juanca. A mi hermano Javier. A Claire. A mis abuelitos. A mis compañeros. A todos los que me apoyaron. A la naturaleza. A la esperanza por la PAZ. v AGRADECIMIENTOS A José L. Linares, por su asesoría, alegría y buena cocina. Al Dr. Pilz, por la tranquilidad. A mis padres, por todo su gran apoyo, soporte, aguante y cariño brindado. A Javier por ser mi hermano. A los clanes Rojas y Muñoz-Reyes, por haberme acogido. A los Babos, por ser un ejemplo de valores. A la Mimi, por su alegría. -

Roadrunner News Newsletter of the Long Beach Cactus Club Founded 1933; Affiliate of the Cactus and Succulent Society of America, Inc
May 2018 Roadrunner News Newsletter of the Long Beach Cactus Club Founded 1933; Affiliate of the Cactus and Succulent Society of America, Inc. Drosanthemum speciosum, photo by Krystoff Przykucki MEETING PROGRAM: Tom Glavich: “The Genus Euphorbia” LOCATION: Rancho Los Alamitos, 6400 Bixby Hill Road, Long Beach, CA 90815. We will meet in the meeting room next to the gift shop. Rancho Los Alamitos is located within Bixby Hill and accessed through the residential security gate at Anaheim and Palo Verde. From the 405 Freeway, exit at Palo Verde Avenue and turn south. From the 605 Freeway, exit at Willow, follow to Palo Verde and turn south. TIME: Sunday, May 6th, 2018 at 1:30 p.m. Setup will be from 12:30 – 1:30. Members will be working in the garden starting at 11 AM. Bring a lunch if you need to. REFRESHMENTS: We will follow the alphabet to determine who is to bring the snacks and finger foods. This month, those with last names starting with the letters A through F are asked to bring the goodies. Please feel free to bring something even if you don’t fall into this group. PLANT-OF-THE-MONTH: Cactus: Echinocactus, Ferocactus, Succulent: Monadenium, Jatropha Descriptions by Scott Bunnell: Echinocactus is a genus of cacti in the subfamily Cactoideae. It and Ferocactus are the two genera of barrel cactus. Members of the genus usually have heavy spination and relatively small flowers. The fruits are copiously woolly, which is one major distinction between Echinocactus and Ferocactus. Propagation is by seed. Perhaps the best known species is the golden barrel (Echinocactus grusonii) from Mexico, an easy-to-grow and widely cultivated plant. -

Bio 308-Course Guide
COURSE GUIDE BIO 308 BIOGEOGRAPHY Course Team Dr. Kelechi L. Njoku (Course Developer/Writer) Professor A. Adebanjo (Programme Leader)- NOUN Abiodun E. Adams (Course Coordinator)-NOUN NATIONAL OPEN UNIVERSITY OF NIGERIA BIO 308 COURSE GUIDE National Open University of Nigeria Headquarters 14/16 Ahmadu Bello Way Victoria Island Lagos Abuja Office No. 5 Dar es Salaam Street Off Aminu Kano Crescent Wuse II, Abuja e-mail: [email protected] URL: www.nou.edu.ng Published by National Open University of Nigeria Printed 2013 ISBN: 978-058-434-X All Rights Reserved Printed by: ii BIO 308 COURSE GUIDE CONTENTS PAGE Introduction ……………………………………......................... iv What you will Learn from this Course …………………............ iv Course Aims ……………………………………………............ iv Course Objectives …………………………………………....... iv Working through this Course …………………………….......... v Course Materials ………………………………………….......... v Study Units ………………………………………………......... v Textbooks and References ………………………………........... vi Assessment ……………………………………………….......... vi End of Course Examination and Grading..................................... vi Course Marking Scheme................................................................ vii Presentation Schedule.................................................................... vii Tutor-Marked Assignment ……………………………….......... vii Tutors and Tutorials....................................................................... viii iii BIO 308 COURSE GUIDE INTRODUCTION BIO 308: Biogeography is a one-semester, 2 credit- hour course in Biology. It is a 300 level, second semester undergraduate course offered to students admitted in the School of Science and Technology, School of Education who are offering Biology or related programmes. The course guide tells you briefly what the course is all about, what course materials you will be using and how you can work your way through these materials. It gives you some guidance on your Tutor- Marked Assignments. There are Self-Assessment Exercises within the body of a unit and/or at the end of each unit. -

University of Florida Thesis Or Dissertation Formatting
SYSTEMATICS OF TRIBE TRICHOCEREEAE AND POPULATION GENETICS OF Haageocereus (CACTACEAE) By MÓNICA ARAKAKI MAKISHI A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2008 1 © 2008 Mónica Arakaki Makishi 2 To my parents, Bunzo and Cristina, and to my sisters and brother. 3 ACKNOWLEDGMENTS I want to express my deepest appreciation to my advisors, Douglas Soltis and Pamela Soltis, for their consistent support, encouragement and generosity of time. I would also like to thank Norris Williams and Michael Miyamoto, members of my committee, for their guidance, good disposition and positive feedback. Special thanks go to Carlos Ostolaza and Fátima Cáceres, for sharing their knowledge on Peruvian Cactaceae, and for providing essential plant material, confirmation of identifications, and their detailed observations of cacti in the field. I am indebted to the many individuals that have directly or indirectly supported me during the fieldwork: Carlos Ostolaza, Fátima Cáceres, Asunción Cano, Blanca León, José Roque, María La Torre, Richard Aguilar, Nestor Cieza, Olivier Klopfenstein, Martha Vargas, Natalia Calderón, Freddy Peláez, Yammil Ramírez, Eric Rodríguez, Percy Sandoval, and Kenneth Young (Peru); Stephan Beck, Noemí Quispe, Lorena Rey, Rosa Meneses, Alejandro Apaza, Esther Valenzuela, Mónica Zeballos, Freddy Centeno, Alfredo Fuentes, and Ramiro Lopez (Bolivia); María E. Ramírez, Mélica Muñoz, and Raquel Pinto (Chile). I thank the curators and staff of the herbaria B, F, FLAS, LPB, MO, USM, U, TEX, UNSA and ZSS, who kindly loaned specimens or made information available through electronic means. Thanks to Carlos Ostolaza for providing seeds of Haageocereus tenuis, to Graham Charles for seeds of Blossfeldia sucrensis and Acanthocalycium spiniflorum, to Donald Henne for specimens of Haageocereus lanugispinus; and to Bernard Hauser and Kent Vliet for aid with microscopy. -

On the Dry Side 2018
APRIL 2018 On the Dry Side Newsletter of the Monterey Bay Area Cactus & Succulent Society Contents Presidents Message Contents .......................................... 1 We are now into the season of spring, when plants –and weeds– President’s Message ........................ 1 grow vigorously and the weather inspires our gardening urges. MBACSS Board Minutes ................. 2 The schedule for our Spring Show & Sale was earlier than usual MBACSS Spring Sale – Pix .............. 3 this year, but the timing apparently was right relative to seasonal April Program ................................. 5 interest in new plants: the turnout was good, and sales seemed MBACSS Calendar 2018 ................. 6 satisfying to our many customers and rewarding for MBACSS’s Additional Resources ....................... 7 propagating members. We’ll learn more about the results as the April Mini-Show Plants ................... 8 financial dust settles. March Mini-Show Winners ............. 9 Many thanks to vendors and other members who supported the Officers & Chairpersons ................... 10 success of this event. We will thank each one during our meeting, What Are They Thinking? ............... 10 but we appreciate in particular Naomi Bloss, Gary Stubblefield and Sarah Martin. Our speaker for the April meeting, Ernesto Sandoval, has a timely topic for this spring season: “Succulent Propagation from Seeds” will provide technical information and horticultural inspiration for members who are already engaged in seed propagation, or would be interested in giving a try to real gardening. Left: Customers at the Spring Show & Sale examining a possible new addition to their garden. Photo by Paul Albert. Save the Date! MBACSS Meets Board Meets Future Meetings April 15, 2018 April 15, 2018 Third Sundays Veterans of Foreign Potluck @ 12:30 Board @ 11:00 Wars, Post 1716 Gathering @ 12:00 Members always 1960 Freedom Blvd. -

Ecology and Conservation of the Cactus Ferruginous Pygmy-Owl in Arizona
United States Department of Agriculture Ecology and Conservation Forest Service Rocky Mountain of the Cactus Ferruginous Research Station General Technical Report RMRS-GTR-43 Pygmy-Owl in Arizona January 2000 Abstract ____________________________________ Cartron, Jean-Luc E.; Finch, Deborah M., tech. eds. 2000. Ecology and conservation of the cactus ferruginous pygmy-owl in Arizona. Gen. Tech. Rep. RMRS-GTR-43. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 68 p. This report is the result of a cooperative effort by the Rocky Mountain Research Station and the USDA Forest Service Region 3, with participation by the Arizona Game and Fish Department and the Bureau of Land Management. It assesses the state of knowledge related to the conservation status of the cactus ferruginous pygmy-owl in Arizona. The population decline of this owl has been attributed to the loss of riparian areas before and after the turn of the 20th century. Currently, the cactus ferruginous pygmy-owl is chiefly found in southern Arizona in xeroriparian vegetation and well- structured upland desertscrub. The primary threat to the remaining pygmy-owl population appears to be continued habitat loss due to residential development. Important information gaps exist and prevent a full understanding of the current population status of the owl and its conservation needs. Fort Collins Service Center Telephone (970) 498-1392 FAX (970) 498-1396 E-mail rschneider/[email protected] Web site http://www.fs.fed.us/rm Mailing Address Publications Distribution Rocky Mountain Research Station 240 W. Prospect Road Fort Collins, CO 80526-2098 Cover photo—Clockwise from top: photograph of fledgling in Arizona by Jean-Luc Cartron, photo- graph of adult ferruginous pygmy-owl in Arizona by Bob Miles, photograph of adult cactus ferruginous pygmy-owl in Texas by Glenn Proudfoot. -

Plethora of Plants - Collections of the Botanical Garden, Faculty of Science, University of Zagreb (2): Glasshouse Succulents
NAT. CROAT. VOL. 27 No 2 407-420* ZAGREB December 31, 2018 professional paper/stručni članak – museum collections/muzejske zbirke DOI 10.20302/NC.2018.27.28 PLETHORA OF PLANTS - COLLECTIONS OF THE BOTANICAL GARDEN, FACULTY OF SCIENCE, UNIVERSITY OF ZAGREB (2): GLASSHOUSE SUCCULENTS Dubravka Sandev, Darko Mihelj & Sanja Kovačić Botanical Garden, Department of Biology, Faculty of Science, University of Zagreb, Marulićev trg 9a, HR-10000 Zagreb, Croatia (e-mail: [email protected]) Sandev, D., Mihelj, D. & Kovačić, S.: Plethora of plants – collections of the Botanical Garden, Faculty of Science, University of Zagreb (2): Glasshouse succulents. Nat. Croat. Vol. 27, No. 2, 407- 420*, 2018, Zagreb. In this paper, the plant lists of glasshouse succulents grown in the Botanical Garden from 1895 to 2017 are studied. Synonymy, nomenclature and origin of plant material were sorted. The lists of species grown in the last 122 years are constructed in such a way as to show that throughout that period at least 1423 taxa of succulent plants from 254 genera and 17 families inhabited the Garden’s cold glass- house collection. Key words: Zagreb Botanical Garden, Faculty of Science, historic plant collections, succulent col- lection Sandev, D., Mihelj, D. & Kovačić, S.: Obilje bilja – zbirke Botaničkoga vrta Prirodoslovno- matematičkog fakulteta Sveučilišta u Zagrebu (2): Stakleničke mesnatice. Nat. Croat. Vol. 27, No. 2, 407-420*, 2018, Zagreb. U ovom članku sastavljeni su popisi stakleničkih mesnatica uzgajanih u Botaničkom vrtu zagrebačkog Prirodoslovno-matematičkog fakulteta između 1895. i 2017. Uređena je sinonimka i no- menklatura te istraženo podrijetlo biljnog materijala. Rezultati pokazuju kako je tijekom 122 godine kroz zbirku mesnatica hladnog staklenika prošlo najmanje 1423 svojti iz 254 rodova i 17 porodica.