A Patient in Whom Self-Terminating Ventricular Fibrillation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -
Atrial Arrhythmia Triggering Electromechanical Dissociation And
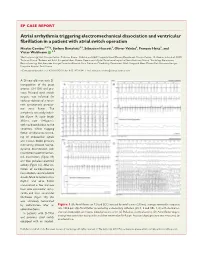
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Cardiac Arrest and Ventricular Fibrillation * a Method of Treatment by Electrical Shock
Thorax: first published as 10.1136/thx.7.3.205 on 1 September 1952. Downloaded from Thorax (1952), 7, 205. CARDIAC ARREST AND VENTRICULAR FIBRILLATION * A METHOD OF TREATMENT BY ELECTRICAL SHOCK BY I. K. R. MCMILLANt F. B. COCKETT, AND P. STYLES Froml the Departnment of Cardiology, tile Professorial Surgical Unit, and tile Departalzent of Phlysical Medicine, St. Thomlas's Hospital, London (RECEIVED FOR PUBLICATION APRIL 30, 1952) Cardiac arrest during operation is a subject of VENTRICULAR FIBRILLATION importance to all surgeons. During intra-thoracic The treatment of ventricular fibrillation bv operations cardiac arrest and ventricular fibrilla- electrical shock therapy is not new and was first tion can be differentiated by direct observation of tried experimentally in 1899 (Prevost and Battelli). the heart. In recent years this method has been extensively Ventricular fibrillation is an incoordinated type investigated in the United States and France of contraction which produces no useful beats. (Kouwenhoven and Kay, 1951; Johnson and The typical rippling movements of the ventricular Kirby, 1951 ; Santy and Marion, 1950). muscle are unmistakable when seen or felt with The treatment of ventricular fibrillation by the hand directly on the heart. The treatment of zlectrical shock gives encouraging results in the copyright. a heart that has stopped is cardiac massage and experimental animal, and may be a useful adjunct adequate oxygenation. The treatment of ventri- in the operating theatre during cardiac and general cular fibrillation which we recommend is, first, surgery. Under these conditions shock therapy massage to maintain the oxygen supply to the can be rapidly applied. Provided that adequate heart through the coronary arteries, followed oxygenation is maintained and direct cardiac mas- http://thorax.bmj.com/ rapidly by electrical shocking to restore normal sage promptly initiated and maintained, shock rhythm therapy can be applied without undue haste. -

The Refractory VF Arrest Patient: a Review of the Current Treatment
1/24/2018 The Refractory VF Arrest Patient: A Review of the Current Treatment Options Marc Conterato, MD, FACEP Office of the Medical Director North Memorial Health Ambulance Service DISCLOSURE STATEMENT • Board Member, MN Resuscitation Consortium - Images of any commercial devices or medications are for illustration purposes only. The inclusion of such images in this presentation does not imply endorsement of any specific device or company. 2 Objectives • Delineate Refractory Ventricular Fibrillation (RVF) • Recurrent VF versus Refractory VF • What is “Electrical Storm” • Current Pre-hospital treatments • Additional hospital treatments • Dual/Double Sequential Defibrillation • ECMO/ECLS-A New Hope? 3 1 1/24/2018 A Standard Scenario • 55 YOF collapses at stop light, and her car rolls into the car in front of her. Airbags do not deploy, and bystanders find her slumped over the steering wheel and pulseless. • First responder/bystanders start CPR, and deliver three AED shocks prior to EMS arrival. • On EMS arrival, a fourth shock is delivered, an IO and alternative airway placed. Automated CPR started. • Epinephrine 2 mg (total) and Amiodarone 300 mg given, and the patient remains in VF. • Patient downtime is now ~25 minutes, and repeat evaluation reveals persistent VF. 4 What are your options • Continue CPR for a total of 30 minutes with recurrent defibrillations, additional epinephrine, bicarbonate and Amiodarone? • “Load and Go” to the local hospital with CPR enroute and continuing the resuscitation? • “Load and Go” to the local CCL (Cardiac Cath Lab) hospital with CPR enroute and continuing the resuscitation, with possible PCI with ongoing CPR? • Call HEMS unit for transfer to CCL hospital, but can they continue effective CPR in the helicopter? • “Load and Go” to a ECMO/ECLS center with CPR enroute and continuing the resuscitation, on the basis they can accept the patient and continue resuscitation? 5 Defining the problem: What is recurrent versus refractory VF (RVF)? • Recurrent VF is a rhythm that terminates with cardioversion, but then recurs rapidly. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803
Department of Health & CMS Manual System Human Services (DHHS) Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803 SUBJECT: Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy I. SUMMARY OF CHANGES: This Change Request (CR) is effective for claims with dates of service on and after October 30, 2013; contractors shall pay claims for Ventricular Assist Devices as destination therapy using the criteria in Pub. 100-03, part 1, section 20.9.1, and Pub. 100-04, Chapter 32, sec. 320. EFFECTIVE DATE: October 30, 2013 *Unless otherwise specified, the effective date is the date of service. IMPLEMENTATION DATE: September 30, 2014 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R=REVISED, N=NEW, D=DELETED-Only One Per Row. R/N/D CHAPTER / SECTION / SUBSECTION / TITLE D 3/90.2.1/Artifiical Hearts and Related Devices R 32/Table of Contents N 32/320/Artificial Hearts and Related Devices N 32/320.1/Coding Requirements for Furnished Before May 1, 2008 N 32/320.2/Coding Requirements for Furnished After May 1, 2008 N 32/320.3/ Ventricular Assist Devices N 32/320.3.1/Postcardiotomy N 32/320.3.2/Bridge-To -Transplantation (BTT) N 32/320.3.3/Destination Therapy (DT) N 32/320.3.4/ Other N 32/320.4/ Replacement Accessories and Supplies for External Ventricular Assist Devices or Any Ventricular Assist Device (VAD) III. -

Persistent Ventricular Fibrillation During Therapeutic Hypothermia and Prolonged High-Dose Vasopressor Therapy: Case Report
ARTICLE IN PRESS The Journal of Emergency Medicine, Vol. xx, No. x, pp. xxx, 2009 Copyright © 2009 Elsevier Inc. Printed in the USA. All rights reserved 0736-4679/09 $–see front matter doi:10.1016/j.jemermed.2009.05.027 Clinical Communications: Adults PERSISTENT VENTRICULAR FIBRILLATION DURING THERAPEUTIC HYPOTHERMIA AND PROLONGED HIGH-DOSE VASOPRESSOR THERAPY: CASE REPORT Joshua C. Poles, BA* Tyler F. Vadeboncoeur, MD† and Bentley J. Bobrow, MD‡ *Kansas City University of Medicine and Biosciences, Kansas City, Missouri, †Department of Emergency Medicine, Mayo Clinic Florida, Jacksonville, Florida, and ‡Department of Emergency Medicine, Maricopa Medical Center, Phoenix, Arizona Reprint Address: [email protected] e Abstract—Background: Recent emphasis on high qual- survival rates remain low, many communities have real- ity prehospital cardiopulmonary resuscitation has resulted ized substantial improvements in meaningful survival in more out-of-hospital cardiac arrest victims surviving to the after OHCA. This has occurred primarily through emergency department. As such, standardized in-hospital emergency medical services (EMS) systems focusing post-cardiac arrest care is necessary to assure optimal neuro- logical recovery. Although therapeutic hypothermia has on delivering high quality cardiopulmonary resuscita- arisen as a key component in the post-cardiac arrest care tion (CPR) (1,2). paradigm, its interaction with other therapies remains Therapeutic hypothermia (TH) is currently an American poorly defined. Objective: The purpose of this communica- Heart Association (AHA) 2005 Guideline IIa therapy for tion is to demonstrate a potential interaction between thera- adult victims who remain unconscious after a ventricular peutic hypothermia and routinely administered resuscitation fibrillation (VF) arrest in the out-of-hospital setting (3). -

Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives
Basic Cardiac Rhythms – Identification and Response Module 1 ANATOMY, PHYSIOLOGY, & ELECTRICAL CONDUCTION Objectives ▪ Describe the normal cardiac anatomy and physiology and normal electrical conduction through the heart. ▪ Identify and relate waveforms to the cardiac cycle. Cardiac Anatomy ▪ 2 upper chambers ▪ Right and left atria ▪ 2 lower chambers ▪ Right and left ventricle ▪ 2 Atrioventricular valves (Mitral & Tricuspid) ▪ Open with ventricular diastole ▪ Close with ventricular systole ▪ 2 Semilunar Valves (Aortic & Pulmonic) ▪ Open with ventricular systole ▪ Open with ventricular diastole The Cardiovascular System ▪ Pulmonary Circulation ▪ Unoxygenated – right side of the heart ▪ Systemic Circulation ▪ Oxygenated – left side of the heart Anatomy Coronary Arteries How The Heart Works Anatomy Coronary Arteries ▪ 2 major vessels of the coronary circulation ▪ Left main coronary artery ▪ Left anterior descending and circumflex branches ▪ Right main coronary artery ▪ The left and right coronary arteries originate at the base of the aorta from openings called the coronary ostia behind the aortic valve leaflets. Physiology Blood Flow Unoxygenated blood flows from inferior and superior vena cava Right Atrium Tricuspid Valve Right Ventricle Pulmonic Valve Lungs Through Pulmonary system Physiology Blood Flow Oxygenated blood flows from the pulmonary veins Left Atrium Mitral Valve Left Ventricle Aortic Valve Systemic Circulation ▪ Blood Flow Through The Heart ▪ Cardiology Rap Physiology ▪ Cardiac cycle ▪ Represents the actual time sequence between -

Update on the Diagnosis and Management of Familial Long QT Syndrome
Heart, Lung and Circulation (2016) 25, 769–776 POSITION STATEMENT 1443-9506/04/$36.00 http://dx.doi.org/10.1016/j.hlc.2016.01.020 Update on the Diagnosis and Management of Familial Long QT Syndrome Kathryn E Waddell-Smith, FRACP a,b, Jonathan R Skinner, FRACP, FCSANZ, FHRS, MD a,b*, members of the CSANZ Genetics Council Writing Group aGreen Lane Paediatric and Congenital Cardiac Services, Starship Children’s Hospital, Auckland New Zealand bDepartment[5_TD$IF] of Paediatrics,[6_TD$IF] Child[7_TD$IF] and[8_TD$IF] Youth[9_TD$IF] Health,[10_TD$IF] University of Auckland, Auckland, New Zealand Received 17 December 2015; accepted 20 January 2016; online published-ahead-of-print 5 March 2016 This update was reviewed by the CSANZ Continuing Education and Recertification Committee and ratified by the CSANZ board in August 2015. Since the CSANZ 2011 guidelines, adjunctive clinical tests have proven useful in the diagnosis of LQTS and are discussed in this update. Understanding of the diagnostic and risk stratifying role of LQTS genetics is also discussed. At least 14 LQTS genes are now thought to be responsible for the disease. High-risk individuals may have multiple mutations, large gene rearrangements, C-loop mutations in KCNQ1, transmembrane mutations in KCNH2, or have certain gene modifiers present, particularly NOS1AP polymorphisms. In regards to treatment, nadolol is preferred, particularly for long QT type 2, and short acting metoprolol should not be used. Thoracoscopic left cardiac sympathectomy is valuable in those who cannot adhere to beta blocker therapy, particularly in long QT type 1. Indications for ICD therapies have been refined; and a primary indication for ICD in post-pubertal females with long QT type 2 and a very long QT interval is emerging. -

Pulseless Ventricular Tachycardia Legend EMR Universal Patient Care SMO EMT Cardiac Arrest SMO Intermediate Paramedic Medical Control
Ventricular Fibrillation/ Pulseless Ventricular Tachycardia Legend EMR Universal Patient Care SMO EMT Cardiac Arrest SMO Intermediate Paramedic Medical Control Not applicable. Emergency Medical Responders and Emergency Medical Technicians are not equipped EMT EMR with ACLS medications and shall treat the patient in accordance with current AHA guidelines and the EMR EMT system Cardiac Arrest SMO. (HPCPR) 1. Continue EMR/EMT care. 2. Evaluate rhythm after 2 minutes CPR. If VF/pulseless VT continue chest compressions while charging and then defibrillate per manufacturer’s recommendations for biphasic monitors or 200J (or 360J for monophasic defibrillators). 3. Immediately resume CPR for 2 minutes and re‐evaluate the patient/rhythm. 4. While performing CPR initiate IV/IO Normal Saline TKO. I 5. Epinephrine (1:10,000) 1 mg IV/IO. I 6. Evaluate rhythm after 2 minutes CPR. If VF/pulseless VT continue chest compressions while charging and then defibrillate per manufacturer’s recommendations for biphasic monitors or 300J (or 360J for monophasic defibrillators). 7. Check rhythm, if still VF/pulseless VT continue, otherwise go to appropriate SMO. CPR for 2 minutes. 8. Evaluate rhythm after 2 minutes CPR. If VF/pulseless VT continue chest compressions while charging and then defibrillate per manufacturer’s recommendations for biphasic monitors or 360J (or 360J for monophasic defibrillators). P 9. Intubate ONLY if it can be accomplished without interruptions to compressions and monitor with ETCO2. Confirm and maintain blind insertion airway device (BIAD) if already inserted. P 10. Epinephrine, 1 mg 1:10,000 IV/IO. Repeat Epinephrine every 3‐5 minutes. 11. CPR for 2 minutes. -

Ventricular Fibrillation Recurrences in Successfully Shocked Out-Of-Hospital Cardiac Arrests
medicina Article Ventricular Fibrillation Recurrences in Successfully Shocked Out-of-Hospital Cardiac Arrests Daniela Aschieri 1 , Federico Guerra 2,* , Valentina Pelizzoni 3, Enrico Paolini 4, Giulia Stronati 2, Luca Moderato 3 , Giulia Losi 1, Paolo Compagnucci 2 , Michela Coccia 1 , Michela Casella 5, Antonio Dello Russo 2, Gust H. Bardy 6 and Alessandro Capucci 2 1 Cardiology Department, Civil Hospital, 29015 Castel San Giovanni, Italy; [email protected] (D.A.); [email protected] (G.L.); [email protected] (M.C.) 2 Department of Biomedical Science and Public Health, Cardiology and Arrhythmology Clinic, University Hospital “Ospedali Riuniti Umberto I-Lancisi-Salesi”, Marche Polytechnic University, 60020 Ancona, Italy; [email protected] (G.S.); [email protected] (P.C.); [email protected] (A.D.R.); [email protected] (A.C.) 3 Cardiology Department, “Guglielmo da Saliceto” Hospital, 29121 Piacenza, Italy; [email protected] (V.P.); [email protected] (L.M.) 4 Cardiology Department, “Ospedali Riuniti Marche Nord”, 61121 Pesaro, Italy; [email protected] 5 Department of Clinical, Special and Dental Sciences, Cardiology and Arrhythmology Clinic, University Hospital “Ospedali Riuniti Umberto I-Lancisi-Salesi”, Marche Polytechnic University, 60020 Ancona, Italy; [email protected] 6 Seattle Institute for Cardiac Research, Seattle, WA 98195, USA; [email protected] * Correspondence: [email protected] Abstract: Background and Objectives: The prognostic impact of ventricular fibrillation (VF) recurrences Citation: Aschieri, D.; Guerra, F.; after a successful shock in out-of-hospital cardiac arrest (OOHCA) is still poorly understood, and Pelizzoni, V.; Paolini, E.; Stronati, G.; some evidence suggests a potential pro-arrhythmic effect of chest compressions in this setting.