Myocarditis and Sudden Death Fact Sheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WPW: WOLFF-PARKINSON-WHITE Syndrome
WPW: WOLFF-PARKINSON-WHITE Syndrome What is Wolff-Parkinson-White Syndrome? Wolff-Parkinson-White Syndrome, or WPW, is named for three physicians who described a syndrome in 1930 in young people with episodes of heart racing and an abnormal pattern on their electrocardiogram (ECG or EKG). Over the next few decades, it was discovered that this ECG pattern and the heart racing was due to an extra electrical pathway in the heart. Thus, WPW is a syndrome associated with an abnormal heart rhythm, or “arrhythmia”. Most people with WPW do not have any other problems with their heart. Normally, the electrical impulses in the heart originate in the atria or top chambers of the heart and spread across the atria. The electrical impulses are then conducted to the ventricles (the pumping/bottom chambers of the heart) through a group of specialized cells called the atrioventricular node or AV node. This is usually the only electrical pathway between the atria and ventricles. In WPW, there is an additional pathway made up of a few extra cells left over from when the heart formed. The conduction of electricity through the heart causes the contractions which are the “heartbeat”. What is WPW Syndrome as opposed to a WPW ECG? A person has WPW Syndrome if they experience symptoms from abnormal conduction through the heart by the WPW pathway. Most commonly, the symptom is heart racing, or “palpitations”. The particular type of arrhythmia in WPW is called “supraventricular tachycardia” or SVT. “Tachycardia” means fast heart rate; “supraventricular” means the arrhythmia requires the cells above the ventricles to be part of the abnormal circuit. -

Cardiac Involvement in COVID-19 Patients: a Contemporary Review
Review Cardiac Involvement in COVID-19 Patients: A Contemporary Review Domenico Maria Carretta 1, Aline Maria Silva 2, Donato D’Agostino 2, Skender Topi 3, Roberto Lovero 4, Ioannis Alexandros Charitos 5,*, Angelika Elzbieta Wegierska 6, Monica Montagnani 7,† and Luigi Santacroce 6,*,† 1 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Coronary Unit and Electrophysiology/Pacing Unit, Cardio-Thoracic Department, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 2 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Cardiac Surgery, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] (A.M.S.); [email protected] (D.D.) 3 Department of Clinical Disciplines, School of Technical Medical Sciences, University of Elbasan “A. Xhuvani”, 3001 Elbasan, Albania; [email protected] 4 AOU Policlinico Consorziale di Bari-Ospedale Giovanni XXIII, Clinical Pathology Unit, Policlinico University Hospital of Bari, 70124 Bari, Italy; [email protected] 5 Emergency/Urgent Department, National Poisoning Center, Riuniti University Hospital of Foggia, 71122 Foggia, Italy 6 Department of Interdisciplinary Medicine, Microbiology and Virology Unit, University of Bari “Aldo Moro”, Piazza G. Cesare, 11, 70124 Bari, Italy; [email protected] 7 Department of Biomedical Sciences and Human Oncology—Section of Pharmacology, School of Medicine, University of Bari “Aldo Moro”, Policlinico University Hospital of Bari, p.zza G. Cesare 11, 70124 Bari, Italy; [email protected] * Correspondence: [email protected] (I.A.C.); [email protected] (L.S.) † These authors equally contributed as co-last authors. Citation: Carretta, D.M.; Silva, A.M.; D’Agostino, D.; Topi, S.; Lovero, R.; Charitos, I.A.; Wegierska, A.E.; Abstract: Background: The widely variable clinical manifestations of SARS-CoV2 disease (COVID-19) Montagnani, M.; Santacroce, L. -

Ischemic Cardiomyopathy: Symptoms, Causes, & Treatment
Ischemic Cardiomyopathy Ischemic cardiomyopathy is a condition that occurs when the heart muscle is weakened due to insufficient blood flow to the heart's muscle. This inhibits the heart's ability to pump blood and can lead to heart failure. What Is Ischemic Cardiomyopathy? Ischemic cardiomyopathy (IC) is a condition that occurs when the heart muscle is weakened. In this condition, the left ventricle, which is the main heart muscle, is usually enlarged and dilated. This condition can be a result of a heart attack or coronary artery disease, a narrowing of the arteries. These narrowed arteries keep blood from reaching portions of your heart. The weakened heart muscle inhibits your heart’s ability to pump blood and can lead to heart failure. Symptoms of IC include shortness of breath, chest pain, and extreme fatigue. If you have IC symptoms, you should seek medical care immediately. Treatment depends on how much damage has been done to your heart. Medications and surgery are often required. You can improve your long-term outlook by making certain lifestyle changes, such as maintaining a healthy diet and avoiding high-risk behaviors, including smoking. Symptoms of Ischemic Cardiomyopathy You can have early-stage heart disease with no symptoms. As the arteries narrow further and blood flow becomes impaired, you may experience a variety of symptoms, including: shortness of breath extreme fatigue dizziness, lightheadedness, or fainting chest pain and pressure (angina) heart palpitations weight gain swelling in the legs and feet (edema) and abdomen difficulty sleeping cough or congestion caused by fluid in the lungs If you have these symptoms, seek emergency medical care or call 9-1-1. -

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Myocarditis and Cardiomyopathy
CE: Tripti; HCO/330310; Total nos of Pages: 6; HCO 330310 REVIEW CURRENT OPINION Myocarditis and cardiomyopathy Jonathan Buggey and Chantal A. ElAmm Purpose of review The aim of this study is to summarize the literature describing the pathogenesis, diagnosis and management of cardiomyopathy related to myocarditis. Recent findings Myocarditis has a variety of causes and a heterogeneous clinical presentation with potentially life- threatening complications. About one-third of patients will develop a dilated cardiomyopathy and the pathogenesis is a multiphase, mutlicompartment process that involves immune activation, including innate immune system triggered proinflammatory cytokines and autoantibodies. In recent years, diagnosis has been aided by advancements in cardiac MRI, and in particular T1 and T2 mapping sequences. In certain clinical situations, endomyocardial biopsy (EMB) should be performed, with consideration of left ventricular sampling, for an accurate diagnosis that may aid treatment and prognostication. Summary Although overall myocarditis accounts for a minority of cardiomyopathy and heart failure presentations, the clinical presentation is variable and the pathophysiology of myocardial damage is unique. Cardiac MRI has significantly improved diagnostic abilities, but endomyocardial biopsy remains the gold standard. However, current treatment strategies are still focused on routine heart failure pharmacotherapies and supportive care or cardiac transplantation/mechanical support for those with end-stage heart failure. Keywords cardiac MRI, cardiomyopathy, endomyocardial biopsy, myocarditis INTRODUCTION prevalence seen in children and young adults aged Myocarditis refers to inflammation of the myocar- 20–30 years [1]. dium and may be caused by infectious agents, systemic diseases, drugs and toxins, with viral infec- CAUSE tions remaining the most common cause in the developed countries [1]. -

ICD-10-CM Documentation and Coding Best Practices
ICD-10-CM Documentation and Coding Best Practices Cardiomyopathy Cardiomyopathy refers to diseases of the heart muscle, which can become enlarged, thick or rigid. In rare cases, cardiac muscle tissue can be replaced with scar tissue. As the condition worsens, the heart becomes weaker and less able to pump blood through the body or to maintain a normal electrical rhythm. Causes Risk factors that can increase the possibility of developing cardiomyopathy include: coronary artery disease, a history of heart attack (s), viral infections that cause heart inflammation, long-term hypertension or alcoholism, obesity, and diabetes to name a few. However, in most cases the exact cause is usually unknown (called primary or idiopathic cardiomyopathy). Symptoms Some patients will never have symptoms; others will not develop them until later in the disease. Symptoms can include: • Fatigue • Shortness of b reath or trouble breathing (dyspnea) • Dizziness, lightheadedness or fainting • Swelling in the ankles, feet, legs, abdomen and neck veins Treatment Treatment depends on the type of cardiomyopathy, the severity of symptoms, and the patient’s age and overall health. • Lifestyle changes can help manage condition(s) that may be causing cardiomyopathy. Recommendations include: o Consuming a heart healthy diet, engaging in physical activity, losing excess weight, giving up smoking, avoiding alcohol and illegal drugs, getting enough sleep, and reducing stress • Medicines may be p rescribed to: o Lower blood pressure (ACE inhibitors, angiotensin II receptor blo ckers, beta blockers, calcium channel blockers ) o Slow the heart rate (beta blockers, calcium channel blockers, digoxin) o Prevent arrhythmias (antiarrhythmics) o Remove excess fluid and sodium (diuretics) o Prevent blood clots (anticoagulants) • Alcohol septal ablation • Surgery o Septal myectomy – option for severe cases of obstructive hypertrophic cardiomyopathy Surgically implanted devices o . -

Myocarditis, Pericarditis and Other Pericardial Diseases
Heart 2000;84:449–454 Diagnosis is easiest during epidemics of cox- GENERAL CARDIOLOGY sackie infections but diYcult in isolated cases. Heart: first published as 10.1136/heart.84.4.449 on 1 October 2000. Downloaded from These are not seen by cardiologists unless they develop arrhythmia, collapse or suVer chest Myocarditis, pericarditis and other pain, the majority being dealt with in the primary care system. pericardial diseases Acute onset of chest pain is usual and may mimic myocardial infarction or be associated 449 Celia M Oakley with pericarditis. Arrhythmias or conduction Imperial College School of Medicine, Hammersmith Hospital, disturbances may be life threatening despite London, UK only mild focal injury, whereas more wide- spread inflammation is necessary before car- diac dysfunction is suYcient to cause symp- his article discusses the diagnosis and toms. management of myocarditis and peri- Tcarditis (both acute and recurrent), as Investigations well as other pericardial diseases. The ECG may show sinus tachycardia, focal or generalised abnormality, ST segment eleva- tion, fascicular blocks or atrioventricular con- Myocarditis duction disturbances. Although the ECG abnormalities are non-specific, the ECG has Myocarditis is the term used to indicate acute the virtue of drawing attention to the heart and infective, toxic or autoimmune inflammation of leading to echocardiographic and other investi- the heart. Reversible toxic myocarditis occurs gations. Echocardiography may reveal segmen- in diphtheria and sometimes in infective endo- -

Review of Systems Form
Child’s name or label Since the last visit, how are your child’s symptoms? Better? Worse? The same? Please describe: Any new health problems, consultations, ER visits, hospital admissions, procedures or surgeries since your last visit? None Evaluations or tests done since the last visit: MRI EEG Blood work Child Study Team evaluation Neuropsychological testing ImPACT test Audiology Nutritionist consultation Questionnaires Physician Consultations:______________________ Other: ____________________ None If your child takes medications, please list the medications and doses here: 1.__________________________________________________ 4.__________________________________________ 2._________________________________________________ 5._________________________________________ 3.__________________________________________________ 6.__________________________________________ PEDS NEURO REVIEW OF SYSTEMS: Please list symptoms your child has since the last visit. Describe “yes” responses. NEUROLOGICAL KNOWN EYE CONDITIONS YES NO ___________________ HYPERACTIVITY YES NO ___________________ EAR, NOSE AND THROAT SEIZURES YES NO ___________________ HEARING LOSS OR DEFICIT YES NO ___________________ FAINTING YES NO ___________________ SLEEP APNEA YES NO ___________________ SNORING YES NO ___________________ CARDIOVASCULAR HEADACHES YES NO ___________________ RAPID OR IRREGULAR HEART BEAT YES NO ___________________ TICS YES NO ___________________ CHEST PAIN OR EXERCISE INTOLERANCE YES NO ___________________ INATTENTION -
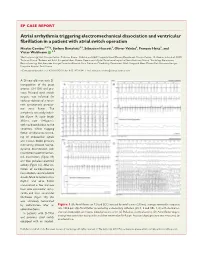
Atrial Arrhythmia Triggering Electromechanical Dissociation And
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

The Pulmonary Manifestations of Left Heart Failure*
The Pulmonary Manifestations of Left Heart Failure* Brian K. Gehlbach, MD; and Eugene Geppert, MD Determining whether a patient’s symptoms are the result of heart or lung disease requires an understanding of the influence of pulmonary venous hypertension on lung function. Herein, we describe the effects of acute and chronic elevations of pulmonary venous pressure on the mechanical and gas-exchanging properties of the lung. The mechanisms responsible for various symptoms of congestive heart failure are described, and the significance of sleep-disordered breathing in patients with heart disease is considered. While the initial clinical evaluation of patients with dyspnea is imprecise, measurement of B-type natriuretic peptide levels may prove useful in this setting. (CHEST 2004; 125:669–682) Key words: Cheyne-Stokes respiration; congestive heart failure; differential diagnosis; dyspnea; pulmonary edema; respiratory function tests; sleep apnea syndromes Abbreviations: CHF ϭ congestive heart failure; CSR-CSA ϭ Cheyne-Stokes respiration with central sleep apnea; CPAP ϭ continuous positive airway pressure; Dlco ϭ diffusing capacity of the lung for carbon monoxide; DM ϭ membrane conductance; FRC ϭ functional residual capacity; OSA ϭ obstructive sleep apnea; TLC ϭ total lung ϭ ˙ ˙ ϭ capacity; VC capillary volume; Ve/Vco2 ventilatory equivalent for carbon dioxide early 5 million Americans have congestive heart For a detailed review of the pathophysiology of N failure (CHF), with 400,000 new cases diag- high-pressure pulmonary edema, the reader is re- nosed each year.1 Unfortunately, despite the consid- ferred to several excellent recent reviews.2–4 erable progress that has been made in understanding the pathophysiology of pulmonary edema, the pul- monary complications of this condition continue to The Pathophysiology of Pulmonary challenge the bedside clinician. -

NIH Public Access Author Manuscript Auton Neurosci
NIH Public Access Author Manuscript Auton Neurosci. Author manuscript; available in PMC 2016 March 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Auton Neurosci. 2015 March ; 188: 86–89. doi:10.1016/j.autneu.2014.11.008. Exercise in the Postural Orthostatic Tachycardia Syndrome Qi Fu and Benjamin D. Levine Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital Dallas, The University of Texas Southwestern Medical Center, Dallas, TX, USA Abstract Patients with the Postural Orthostatic Tachycardia Syndrome (POTS) have orthostatic intolerance, as well as exercise intolerance. Peak oxygen uptake (VO2peak) is generally lower in these patients compared with healthy sedentary individuals, suggesting a lower physical fitness level. During acute exercise, POTS patients have an excessive increase in heart rate and reduced stroke volume for each level of absolute workload; however, when expressed at relative workload (%VO2peak), there is no difference in the heart rate response between patients and healthy individuals. The relationship between cardiac output and VO2 is similar between POTS patients and healthy individuals. Short-term (i.e., 3 months) exercise training increases cardiac size and mass, blood volume, and VO2peak in POTS patients. Exercise performance is improved after training. Specifically, stroke volume is greater and heart rate is lower at any given VO2 during exercise after training versus before training. Peak heart rate is the same but peak stroke volume and cardiac output are greater after training. Heart rate recovery from peak exercise is significantly faster after training, indicating an improvement in autonomic circulatory control. These results suggest that patients with POTS have no intrinsic abnormality of heart rate regulation during exercise.