The Pulmonary Manifestations of Left Heart Failure*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lung Function Studies in Diagnostics and Follow-Up of Pulmonary Sarcoidosis
Lung Function Studies in Diagnostics and Follow-up of Pulmonary Sarcoidosis By INGELA BRÅDVIK Lund 1994 From the Department of Lung Medicine and the Department of Clinical Physiology University of Lund, Sweden Lung Function Studies in Diagnostics and Follow-up of Pulmonary Sarcoidosis Ingela Brådvik Lund 1994 Organization Document name LUND UNIVERSITY DOCTORAL DISSERTATION Department of Lung Medicine Date of issue University Hospital 94 06 09 S-22I 85 Lund CODEN: ISRN LUMEDW/MELL- - 1007- - SE Authorfj) Sponsoring organization Ingela Brådvik The Swedish Heart Lung Foundation Title and subtitle Lung function studies in diagnostics and follow-up of pulmonary sarcoidosis Abstract In 66 patients the relationship between lung volumes and lung mechanics in pulmonary sarcoidosis was investigated Lung volumes, static lung mechanics, lung resistance, dynamic lung mechanics and arterial blood gases at rest and during exercise were obtained. Fifteen functionally compromised patients received steroids during one year. They were re-investigated during the treatment and at a follow-up after an average of 7 years. In another 41 patients with newly diagnosed sarcoidosis, the kinetics of the lung clearance of ^9mTc-DTPA measured over 180 minutes was explored, and compared to kinetics in healthy smokers. The relationship between lung clearance and lung volumes, lung mechanics, arterial blood gases and disease activity assessed with serum angiotensin-converting enzyme and "'Ga scintigraphy was studied. Reduced lung volumes and compliance, increased resistance and decreased arterial oxygen tension were common. Vital i capacity (VC), and changes of VC at follow-up, corresponded to the slope of the static elastic pressure/volume curve, . and to the variation of it. -

Bronchodilators Accelerate the Dynamics of Muscle O2 Delivery
Chronic obstructive pulmonary disease Bronchodilators accelerate the dynamics of muscle Thorax: first published as 10.1136/thx.2009.120857 on 13 July 2010. Downloaded from O2 delivery and utilisation during exercise in COPD Danilo C Berton,1 Priscila B Barbosa,1 Luciana S Takara,1 Gaspar R Chiappa,1 Ana Cristina B Siqueira,1 Daniela M Bravo,1 Leonardo F Ferreira,2 J Alberto Neder1 See Editorial, p 573 ABSTRACT (QT), suggesting an important role for the central Background Expiratory flow limitation and lung cardiovascular adjustments in setting the limits of < Supplementary methods are hyperinflation promote cardiocirculatory perturbations increase in peripheral QO at the onset of exercise. published online only. To view 2 these files please visit the that might impair O2 delivery to locomotor muscles in Further experimental evidence for this contention journal online (http://thorax.bmj. patients with chronic obstructive pulmonary disease was obtained in a subsequent study in which com). (COPD). The hypothesis that decreases in lung hyperinflation a strategy aimed to reduce the resistive work of 1Pulmonary Function and Clinical after the inhalation of bronchodilators would improve breathing and dynamic hyperinflation (heliox) Exercise Physiology Unit skeletal muscle oxygenation during exercise was tested. simultaneously accelerated the dynamics of QTand (SEFICE), Division of Respiratory 5 Methods Twelve non- or mildly hypoxaemic males QO2 in patients with moderate to severe COPD. Diseases, Department of (forced expiratory volume in 1 s (FEV1)¼38.5612.9% -

Final Program for the ATS International Conference Is Available in Printed and Digital Format
WELCOME TO ATS 2017 • WASHINGTON, DC Welcome to ATS 2017 Welcome to Washington, DC for the 2017 American Thoracic Society International Conference. The conference, which is expected to draw more than 15,000 investigators, educators, and clinicians, is truly the destination for pediatric and adult pulmonary, critical care, and sleep medicine professionals at every level of their careers. The conference is all about learning, networking and connections. Because it engages attendees across many disciplines and continents, the ATS International Conference draws a large, diverse group of participants, a dedicated and collegial community that inspires each of us to make a difference in patients’ lives, now and in the future. By virtue of its size — ATS 2017 features approximately 6,700 original research projects and case reports, 500 sessions, and 800 speakers — participants can attend David Gozal, MD sessions and special events from early morning to the evening. At ATS 2017 there will be something for President everyone. American Thoracic Society Don’t miss the following important events: • Opening Ceremony featuring a keynote presentation by Nobel Laureate James Heckman, PhD, MA, from the Center for the Economics of Human Development at the University of Chicago. • Ninth Annual ATS Foundation Research Program Benefit honoring David M. Center, MD, with the Foundation’s Breathing for Life Award on Saturday. • ATS Diversity Forum will feature Eliseo J. Pérez-Stable, MD, Director, National Institute on Minority Health and Health Disparities at the National Institutes of Health. • Keynote Series highlight state of the art lectures on selected topics in an unopposed format to showcase major discoveries in pulmonary, critical care and sleep medicine. -

Effect of Heliox Breathing on Flow Limitation in Chronic Heart Failure Patients
Eur Respir J 2009; 33: 1367–1373 DOI: 10.1183/09031936.00117508 CopyrightßERS Journals Ltd 2009 Effect of heliox breathing on flow limitation in chronic heart failure patients M. Pecchiari*, T. Anagnostakos#, E. D’Angelo*, C. Roussos#, S. Nanas# and A. Koutsoukou# ABSTRACT: Patients with chronic heart failure (CHF) exhibit orthopnoea and tidal expiratory flow AFFILIATIONS limitation in the supine position. It is not known whether the flow-limiting segment occurs in the *Istituto di Fisiologia Umana I, Universita` degli Studi di Milano, peripheral or central part of the tracheobronchial tree. The location of the flow-limiting segment Milan, Italy, and can be inferred from the effects of heliox (80% helium/20% oxygen) administration. If maximal #Dept of Critical Care and Pulmonary expiratory flow increases with this low-density mixture, the choke point should be located in the Services, Evangelismos General central airways, where the wave-speed mechanism dominates. If the choke point were located in Hospital, Medical School, University of Athens, Athens, Greece. the peripheral airways, where maximal flow is limited by a viscous mechanism, heliox should have no effect on flow limitation and dynamic hyperinflation. CORRESPONDENCE Tidal expiratory flow limitation, dynamic hyperinflation and breathing pattern were assessed in M. Pecchiari 14 stable CHF patients during air and heliox breathing at rest in the sitting and supine position. Istituto di Fisiologia Umana I via L. Mangiagalli 32 No patient was flow-limited in the sitting position. In the supine posture, eight patients exhibited 20133 Milan tidal expiratory flow limitation on air. Heliox had no effect on flow limitation and dynamic Italy hyperinflation and only minor effects on the breathing pattern. -

The Measurement of Pulmonary Diffusing Capacity for Carbon Monoxide by a Rebreathing Method
THE MEASUREMENT OF PULMONARY DIFFUSING CAPACITY FOR CARBON MONOXIDE BY A REBREATHING METHOD Benjamin M. Lewis, … , Ernest J. Hayford-Welsing, Erma Flaherty J Clin Invest. 1959;38(11):2073-2086. https://doi.org/10.1172/JCI103985. Research Article Find the latest version: https://jci.me/103985/pdf THE MEASUREMENT OF PULMONARY DIFFUSING CAPACITY FOR CARBON MONOXIDE BY A REBREATHING METHOD*t By BENJAMIN M. LEWIS, TAI-HON LIN, FRANCES E. NOET AND ERNEST J. HAYFORD-WELSING§ WITH THE TECHNICAL ASSISTANCE OF ERMA FLAHERTY (From the Pulmonary Function Laboratories, Departments of Medicine, Wayne State University College of Medicine, and City of Detroit Receiving Hospital, Detroit, Mich.) (Submitted for publication February 18, 1959; accepted June 19, 1959) The pulmonary diffusing capacity for oxygen capacity and one second vital capacity are first determined. is of great physiological and clinical sig- The analyzer circuit of the apparatus is then flushed with (DLo,) tank oxygen. A sealed bag containing a volume of 0.3 nificance (1). Its measurement, however, is rela- per cent CO and 10 per cent He in air (or in oxygen)2 tively complex (2). Pulmonary diffusing capac- equal to the subject's one second vital capacity is attached ity for carbon monoxide (DLco) which, it is to the three-way tap, the clamps on the bag are removed usually assumed,' can be converted to DL02 from and the bag and analyzer circuit mixed by the pump.3 the known solubilities and molecular weights of A bag-in-box device attached to a spirometer has been found convenient for filling the bag. -

Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting
Percutaneous Mitral Valve Therapies: State of the Art in 2020 LA ACP Annual Meeting Steven R Bailey MD MSCAI, FACC, FAHA,FACP Professor and Chair, Department of Medicine Malcolm Feist Chair of Interventional Cardiology LSU Health Shreveport Professor Emeritus, UH Health San Antonio [email protected] SRB March 2020 Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship Company • Grant/Research Support • None • Consulting Fees/Honoraria • BSCI, Abbot DSMB • Intellectual Property Rights • UTHSCSA • Other Financial Benefit • CCI Editor In Chief SRB March 2020 The 30,000 Ft View Maria SRB March 2020 SRB March 2020 Mitral Stenosis • The most common etiology of MS is rheumatic fever, with a latency of approximately 10 to 20 years after the initial streptococcal infection. Symptoms usually appear in adulthood • Other etiologies are rare but include: congenital MS radiation exposure atrial myxoma mucopolysaccharidoses • MS secondary to calcific annular disease is increasingly seen in elderly patients, and in patients with advanced chronic kidney disease. SRB March 2020 Mitral Stenosis • Mitral stenosis most commonly results from rheumatic heart disease fusion of the valve leaflet cusps at the commissures thickening and shortening of the chordae calcium deposition within the valve leaflets • Characteristic “fish-mouth” or “hockey stick” appearance on the echocardiogram (depending on view) SRB March 2020 Mitral Stenosis: Natural History • The severity of symptoms depends primarily on the degree of stenosis. • Symptoms often go unrecognized by patient and physician until significant shortness of breath, hemoptysis, or atrial fibrillation develops. -

Making the Diagnosis of Asthma
Making the Diagnosis of Asthma Meredith C McCormack MD MHS and Paul L Enright MD Introduction Signs and Symptoms Differential Diagnosis Diagnostic Testing Spirometry Bronchodilator Response Testing Inhalation Challenge Test Radioallergosorbent Test and Allergen Skin Test Exhaled Nitric Oxide Radiographic Imaging Asthma Versus Chronic Obstructive Pulmonary Disease Asthma Severity Summary: Take-Home Messages for the Respiratory Therapist Diagnostic tests can only increase or decrease the probability of the asthma diagnosis, so a thorough history is very important. In patients with asthma-like symptoms, spirometric evidence of airway obstruction plus a large bronchodilator response makes asthma much more likely. However, nor- mal spirometry is common in patients with mild asthma who are not symptomatic at the time of testing, and patients with poorly controlled asthma may lack substantial bronchodilator response. Inhalation challenge test often helps confirm asthma in patients with normal spirometry. Adult smokers with intermittent respiratory symptoms may have either asthma or chronic obstructive pulmonary disease (COPD). Normal post-bronchodilator spirometry rules out COPD. In patients with airway obstruction, a low diffusing capacity of the lung for carbon monoxide increases the probability of COPD and makes asthma much less likely. A high exhaled nitric oxide level makes allergic asthma more likely. Response to inhaled corticosteroids makes asthma more likely and COPD less likely. Key words: asthma, spirometry, methacholine, bronchodilator, pulmonary function, diagnosis. [Respir Care 2008;53(5):583–590. © 2008 Daedalus Enterprises] Meredith C McCormack MD MHS is affiliated with the Division of consulting on spirometry quality-assurance programs for a phase-3 clin- Pulmonary and Critical Care Medicine, Johns Hopkins School of Med- ical trial of varenicline for smoking cessation. -

Currentstateofknowledgeonaetiol
European Heart Journal (2013) 34, 2636–2648 ESC REPORT doi:10.1093/eurheartj/eht210 Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases Downloaded from Alida L. P. Caforio1†*, Sabine Pankuweit2†, Eloisa Arbustini3, Cristina Basso4, Juan Gimeno-Blanes5,StephanB.Felix6,MichaelFu7,TiinaHelio¨ 8, Stephane Heymans9, http://eurheartj.oxfordjournals.org/ Roland Jahns10,KarinKlingel11, Ales Linhart12, Bernhard Maisch2, William McKenna13, Jens Mogensen14, Yigal M. Pinto15,ArsenRistic16, Heinz-Peter Schultheiss17, Hubert Seggewiss18, Luigi Tavazzi19,GaetanoThiene4,AliYilmaz20, Philippe Charron21,andPerryM.Elliott13 1Division of Cardiology, Department of Cardiological Thoracic and Vascular Sciences, University of Padua, Padova, Italy; 2Universita¨tsklinikum Gießen und Marburg GmbH, Standort Marburg, Klinik fu¨r Kardiologie, Marburg, Germany; 3Academic Hospital IRCCS Foundation Policlinico, San Matteo, Pavia, Italy; 4Cardiovascular Pathology, Department of Cardiological Thoracic and Vascular Sciences, University of Padua, Padova, Italy; 5Servicio de Cardiologia, Hospital U. Virgen de Arrixaca Ctra. Murcia-Cartagena s/n, El Palmar, Spain; 6Medizinische Klinik B, University of Greifswald, Greifswald, Germany; 7Department of Medicine, Heart Failure Unit, Sahlgrenska Hospital, University of Go¨teborg, Go¨teborg, Sweden; 8Division of Cardiology, Helsinki University Central Hospital, Heart & Lung Centre, -

Performance Characteristics in Idiopathic Pulmonary Fibrosis
ORIGINAL ARTICLE INTERSTITIAL LUNG DISEASE AND RADIOLOGY Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis Stephen M. Humphries1, Jeffrey J. Swigris2, Kevin K. Brown2, Matthew Strand3, Qi Gong4, John S. Sundy4, Ganesh Raghu5, Marvin I. Schwarz6, Kevin R. Flaherty7, Rohit Sood8, Thomas G. O’Riordan4 and David A. Lynch1 Affiliations: 1Dept of Radiology, National Jewish Health, Denver, CO, USA. 2Division of Pulmonary and Critical Care Medicine, National Jewish Health, Denver, CO, USA. 3Division of Biostatistics and Bioinformatics, National Jewish Health, Denver, CO, USA. 4Gilead Sciences Inc., Foster City, CA, USA. 5Center for Interstitial Lung Diseases, Dept of Medicine, University of Washington, Seattle, WA, USA. 6Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado, Aurora, CO, USA. 7Division of Pulmonary and Critical Care Medicine, University of Michigan, Ann Arbor, MI, USA. 8PAREXEL International, Billerica, MA, USA. Correspondence: Stephen M. Humphries, Quantitative Imaging Laboratory, Dept of Radiology, National Jewish Health, 1400 Jackson Street, Denver, CO 80206-2761, USA. E-mail: [email protected] @ERSpublications In subjects with IPF, quantification of lung fibrosis extent on HRCT using data-driven texture analysis shows acceptable performance characteristics and minimal clinically important difference in the range of 3.4–6.4% http://ow.ly/fFNc30lfAGh Cite this article as: Humphries SM, Swigris JJ, Brown KK, et al. Quantitative high-resolution computed tomography fibrosis score: performance characteristics in idiopathic pulmonary fibrosis. Eur Respir J 2018; 52: 1801384 [https://doi.org/10.1183/13993003.01384-2018]. ABSTRACT We evaluated performance characteristics and estimated the minimal clinically important difference (MCID) of data-driven texture analysis (DTA), a high-resolution computed tomography (HRCT)-derived measurement of lung fibrosis, in subjects with idiopathic pulmonary fibrosis (IPF). -
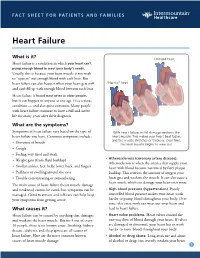
Heart Failure
FACT SHEET FOR PATIENTS AND FAMILIES Heart Failure What is it? Enlarged heart Heart failure is a condition in which your heart can’t pump enough blood to meet your body’s needs. Usually, this is because your heart muscle is too weak to “squeeze” out enough blood with each beat. But heart failure can also happen when your heart gets stiff “Normal” heart and can’t fill up with enough blood between each beat. Heart failure is found most often in older people, but it can happen to anyone at any age. It’s a serious condition — and also quite common. Many people with heart failure continue to have a full and active life for many years after their diagnosis. What are the symptoms? Symptoms of heart failure vary based on the type of With heart failure, initial damage weakens the heart failure you have. Common symptoms include: heart muscle. This makes your heart beat faster, and the muscle stretches or thickens. Over time, • Shortness of breath the heart muscle begins to wear out. • Cough • Feeling very tired and weak • Atherosclerosis (coronary artery disease). • Weight gain (from fluid buildup) Atherosclerosis is when the arteries that supply your • Swollen ankles, feet, belly, lower back, and fingers heart with blood become narrowed by fatty plaque • Puffiness or swelling around the eyes buildup. This restricts the amount of oxygen your • Trouble concentrating or remembering heart gets and weakens the muscle. It can also cause a heart attack, which can damage your heart even more. The main cause of heart failure (heart muscle damage and weakness) cannot be cured, but symptoms can be • High blood pressure (hypertension). -

Heart Disease and Diseases of the Circulatory System in Westchester
Westchester County 2016.01 Department of Health KEEP HEALTHY @wchealthdept AND Community Health Assessment Data Update GET #keephealthy THE STATS Heart Disease and Diseases of the Circulatory System in Westchester In this issue: Heart disease as a Heart disease is the number one cause of death in Westchester County. leading cause of death in Westchester county In 2012, heart disease accounted for 2,113 deaths or 31% of all deaths Deaths due to heart disease across different in the county. Adding in 490 deaths due to stroke and other diseases population and risk of the circulatory system, total deaths from circulatory disease are groups 60% higher than the next leading cause of death - cancer. Hospitalizations due to cardiovascular disease- related conditions, Selected Causes of Death in Westchester County, 2012 including diseases of the heart Emergency room visits 2% 3% 7% due to cardiovascular disease-related 3% conditions Selected risk factors 4% that contribute to Heart Disease, cardiovascular disease 4% in Westchester county 31% 5% 9% Cerebrovascular Jiali Li, Ph.D. Director of Disease (Stroke), Research & Evaluation Neoplasms 5% Planning & Evaluation (Cancer), 24% Other Circulatory, Renee Recchia, MPH 3% Acting Deputy Commissioner of Administration Heart Disease Cerebrovascular Disease (Stroke) Project Staff: Other Circulatory Neoplasms (Cancer) Bonnie Lam, MPH Respiratory Diseases External Causes (e.g. accidents) Medical Data Analyst Communicable Diseases Nervous System Diseases Milagros Venuti, MPA Digestive System Diseases -

Cardiovascular Disease: a Costly Burden for America. Projections
CARDIOVASCULAR DISEASE: A COSTLY BURDEN FOR AMERICA PROJECTIONS THROUGH 2035 CARDIOVASCULAR DISEASE: A COSTLY BURDEN FOR AMERICA — PROJECTIONS THROUGH 2035 american heart association CVD Burden Report CVD Burden association heart american table of contents INTRODUCTION ...................................................................................5 ABOUT THIS STUDY ................................................................................................... 6 WHAT IS CVD? ......................................................................................................... 6 Atrial Fibrillation Congestive Heart Failure Coronary Heart Disease High Blood Pressure Stroke PROJECTIONS: PREVALENCE OF CVD .............................................................7 Latest Projections Age, Race, Sex – Differences That Matter PROJECTIONS: COSTS OF CVD ................................................................. 8-11 The Cost Generators: Aging Baby Boomers Medical Costs Breakdown Direct Costs + Indirect Costs RECOMMENDATIONS .............................................................................13-14 Research Prevention Affordable Health Care 3 CARDIOVASCULAR DISEASE: A COSTLY BURDEN FOR AMERICA — PROJECTIONS THROUGH 2035 american heart association CVD Burden Report CVD Burden association heart american Introduction Cardiovascular disease (CVD) has been the leading killer In addition, CVD has become our nation’s costliest chronic of Americans for decades. In years past, a heart attack disease. In 2014, stroke and heart