Epicardial Exposure Provided by a Novel Thoracoscopic Pericardectomy Technique Compared to Standard Pericardial Window
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non Commercial Use Only
Cardiogenetics 2017; volume 7:6304 Sudden death in a young patient with atrial fibrillation Case Report Correspondence: María Angeles Espinosa Castro, Inherited Cardiovascular Disease A 22-year-old man suffered a sudden Program, Cardiology Department, Gregorio María Tamargo, cardiac arrest without previous symptoms Marañón Hospital, Dr. Esquerdo, 46, 28007, María Ángeles Espinosa, while he was at rest, waiting for a subway Madrid, Spain. Víctor Gómez-Carrillo, Miriam Juárez, train. Cardiopulmonary resuscitation was Tel.: +34.91.586.82.90. immediately started using an Automated E-mail: [email protected] Francisco Fernández-Avilés, External Defibrillation that identified the Raquel Yotti Key words: KCNQ1; mutation; channelopa- presence of ventricular fibrillation and thy; sudden cardiac death; atrial fibrillation. Inherited Cardiovascular Disease delivered a shock. Return of spontaneous Program, Cardiology Department, circulation was achieved after three Contributions: MT, acquisition and interpreta- Gregorio Marañón Hospital, Madrid, attempts, being atrial fibrillation (AF) the tion of data for the work, ensuring that ques- Spain patient’s rhythm at this point (Figure 1). tions related to the accuracy or integrity of any He was admitted to our Cardiovascular part of the work is appropriately investigated Intensive Care Unit and therapeutic and resolved; MAE, conception of the work, hypothermia was performed over a period critical revision of the intellectual content, final approval of the version to be published, Abstract of 24 h. After completing hypothermia, ensuring that questions related to the accuracy rewarming, and another 24 h of controlled of any part of the work is appropriately inves- Sudden cardiac death (SCD) in young normothermia the patient awakened with no tigated and resolved; VG-C, acquisition and patients without structural heart disease is residual neurologic damage. -

Median Sternotomy - Gold Standard Incision for Cardiac Surgeons
J Clin Invest Surg. 2016; 1(1): 33-40 doi: 10.25083/2559.5555.11.3340 Technique Median sternotomy - gold standard incision for cardiac surgeons Radu Matache1,2, Mihai Dumitrescu2, Andrei Bobocea2, Ioan Cordoș1,2 1Carol Davila University, Department of Thoracic Surgery, Bucharest, Romania 2Marius Nasta Clinical Hospital, Department of Thoracic Surgery, Bucharest, Romania Abstract Sternotomy is the gold standard incision for cardiac surgeons but it is also used in thoracic surgery especially for mediastinal, tracheal and main stem bronchus surgery. The surgical technique is well established and identification of the correct anatomic landmarks, midline tissue preparation, osteotomy and bleeding control are important steps of the procedure. Correct sternal closure is vital for avoiding short- and long-term morbidity and mortality. The two sternal halves have to be well approximated to facilitate healing of the bone and to avoid instability, which is a risk factor for wound infection. New suture materials and techniques would be expected to be developed to further improve the patients evolution, in respect to both immediate postoperative period and long-term morbidity and mortality Keywords: median sternotomy, cardiac surgeons, thoracic surgery, technique Acne conglobata is a rare, severe form of acne vulgaris characterized by the presence of comedones, papules, pustules, nodules and sometimes hematic or meliceric crusts, located on the face, trunk, neck, arms and buttocks.Correspondence should be addressed to: Mihai Dumitrescu; e-mail: [email protected] Case Report We report the case of a 16 year old Caucasian female patient from the urban area who addressed our dermatology department for erythematous, edematous plaques covered by pustules and crusts, located on the Radu Matache et al. -
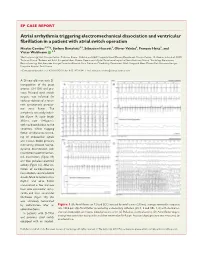
Atrial Arrhythmia Triggering Electromechanical Dissociation And
EP CASE REPORT ....................................................................................................................................................... Atrial arrhythmia triggering electromechanical dissociation and ventricular fibrillation in a patient with atrial switch operation Nicolas Combes1,2,3*, Stefano Bartoletti1,2,Se´bastien Hascoet€ 3, Olivier Vahdat2, Franc¸ois Heitz2, and Victor Waldmann 4,5 1Electrophysiology Unit, Clinique Pasteur, Toulouse, France; 2Pediatric and Adult Congenital Heart Disease Department, Clinique Pasteur, 45, Avenue de Lombez, 31076 Toulouse, France; 3Pediatric and Adult Congenital Heart Disease Department, Hoˆpital Marie Lannelongue, Le Plessis-Robinson, France; 4Cardiology Department, Electrophysiology Unit, European Georges Pompidou Hospital, Paris, France; and 5Cardiology Department, Adult Congenital Heart Disease Unit, European Georges Pompidou Hospital, Paris, France * Corresponding author. Tel: 133 562213131; fax: 133 562211641. E-mail address: [email protected] A 26-year-old man with D- transposition of the great arteries (D-TGA) and pre- vious Mustard atrial switch surgery was referred for catheter ablation of a recur- rent symptomatic paroxys- mal atrial flutter. The arrhythmia was easily induci- ble (Figure 1A, cycle length 310 ms, rate 194 b.p.m.), with rapid conduction to the ventricles. While mapping flutter, simultaneous record- ing of endocardial signals and invasive blood pressure monitoring showed haemo- dynamic deterioration with intermittent electromechan- ical dissociation (Figure 1B) and then pulseless electrical activity (Figure 1C). After ini- tiation of cardiopulmonary resuscitation, several electric shocks failed to restore sinus rhythm and atrial flutter transitioned a few minutes later into ventricular tachy- cardia and then ventricular fibrillation (Figure 1D); this was ultimately terminated by defibrillation after an Figure 1 (A) Atrial flutter on 12-lead ECG induced by atrial bursts (240 ms), average ventricular response adrenaline bolus. -

Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: the Role of Multimodality Imaging to Detect High-Risk Features
diagnostics Review Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality Imaging to Detect High-Risk Features Anna Giulia Pavon 1,2,*, Pierre Monney 1,2,3 and Juerg Schwitter 1,2,3 1 Cardiac MR Center (CRMC), Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland; [email protected] (P.M.); [email protected] (J.S.) 2 Cardiovascular Department, Division of Cardiology, Lausanne University Hospital (CHUV), 1100 Lausanne, Switzerland 3 Faculty of Biology and Medicine, University of Lausanne (UniL), 1100 Lausanne, Switzerland * Correspondence: [email protected]; Tel.: +41-775-566-983 Abstract: Mitral valve prolapse (MVP) was first described in the 1960s, and it is usually a benign condition. However, a subtype of patients are known to have a higher incidence of ventricular arrhythmias and sudden cardiac death, the so called “arrhythmic MVP.” In recent years, several studies have been published to identify the most important clinical features to distinguish the benign form from the potentially lethal one in order to personalize patient’s treatment and follow-up. In this review, we specifically focused on red flags for increased arrhythmic risk to whom the cardiologist must be aware of while performing a cardiovascular imaging evaluation in patients with MVP. Keywords: mitral valve prolapse; arrhythmias; cardiovascular magnetic resonance Citation: Pavon, A.G.; Monney, P.; Schwitter, J. Mitral Valve Prolapse, Arrhythmias, and Sudden Cardiac Death: The Role of Multimodality 1. Mitral Valve and Arrhythmias: A Long Story Short Imaging to Detect High-Risk Features. In the recent years, the scientific community has begun to pay increasing attention Diagnostics 2021, 11, 683. -

Cardiac Arrest and Ventricular Fibrillation * a Method of Treatment by Electrical Shock
Thorax: first published as 10.1136/thx.7.3.205 on 1 September 1952. Downloaded from Thorax (1952), 7, 205. CARDIAC ARREST AND VENTRICULAR FIBRILLATION * A METHOD OF TREATMENT BY ELECTRICAL SHOCK BY I. K. R. MCMILLANt F. B. COCKETT, AND P. STYLES Froml the Departnment of Cardiology, tile Professorial Surgical Unit, and tile Departalzent of Phlysical Medicine, St. Thomlas's Hospital, London (RECEIVED FOR PUBLICATION APRIL 30, 1952) Cardiac arrest during operation is a subject of VENTRICULAR FIBRILLATION importance to all surgeons. During intra-thoracic The treatment of ventricular fibrillation bv operations cardiac arrest and ventricular fibrilla- electrical shock therapy is not new and was first tion can be differentiated by direct observation of tried experimentally in 1899 (Prevost and Battelli). the heart. In recent years this method has been extensively Ventricular fibrillation is an incoordinated type investigated in the United States and France of contraction which produces no useful beats. (Kouwenhoven and Kay, 1951; Johnson and The typical rippling movements of the ventricular Kirby, 1951 ; Santy and Marion, 1950). muscle are unmistakable when seen or felt with The treatment of ventricular fibrillation by the hand directly on the heart. The treatment of zlectrical shock gives encouraging results in the copyright. a heart that has stopped is cardiac massage and experimental animal, and may be a useful adjunct adequate oxygenation. The treatment of ventri- in the operating theatre during cardiac and general cular fibrillation which we recommend is, first, surgery. Under these conditions shock therapy massage to maintain the oxygen supply to the can be rapidly applied. Provided that adequate heart through the coronary arteries, followed oxygenation is maintained and direct cardiac mas- http://thorax.bmj.com/ rapidly by electrical shocking to restore normal sage promptly initiated and maintained, shock rhythm therapy can be applied without undue haste. -

Closed Mitral Commissurotomy—A Cheap, Reproducible and Successful Way to Treat Mitral Stenosis
149 Editorial Closed mitral commissurotomy—a cheap, reproducible and successful way to treat mitral stenosis Manuel J. Antunes Clinic of Cardiothoracic Surgery, Faculty of Medicine, University of Coimbra, Coimbra, Portugal Correspondence to: Prof. Manuel J. Antunes. Faculty of Medicine, University of Coimbra, 3000-075 Coimbra, Portugal. Email: [email protected]. Provenance and Peer Review: This article was commissioned by the Editorial Office, Journal of Thoracic Disease. The article did not undergo external peer review. Comment on: Xu A, Jin J, Li X, et al. Mitral valve restenosis after closed mitral commissurotomy: case discussion. J Thorac Dis 2019;11:3659-71. Submitted Oct 23, 2019. Accepted for publication Nov 29, 2019. doi: 10.21037/jtd.2019.12.118 View this article at: http://dx.doi.org/10.21037/jtd.2019.12.118 In the August issue of the Journal, Xu et al. (1), from Bayley (4,5) and then became widely accepted. Subsequently, China, discuss the case of a patient who had a successful the technique of CMC suffered several modifications, both reoperation for restenosis of the mitral valve performed in the way the mitral valve was accessed and split. Several 30 years after closed mitral commissurotomy (CMC). instruments were created to facilitate the opening of the The specific aspects of this case were most appropriately commissures, culminating with the development of the commented by several experienced surgeons from different Tubbs dilator, which became the standard instrument for parts of the world. I was now invited by the Editor of this the procedure (Figure 1). Journal to write a Comment on this paper and its subject. -

The Refractory VF Arrest Patient: a Review of the Current Treatment
1/24/2018 The Refractory VF Arrest Patient: A Review of the Current Treatment Options Marc Conterato, MD, FACEP Office of the Medical Director North Memorial Health Ambulance Service DISCLOSURE STATEMENT • Board Member, MN Resuscitation Consortium - Images of any commercial devices or medications are for illustration purposes only. The inclusion of such images in this presentation does not imply endorsement of any specific device or company. 2 Objectives • Delineate Refractory Ventricular Fibrillation (RVF) • Recurrent VF versus Refractory VF • What is “Electrical Storm” • Current Pre-hospital treatments • Additional hospital treatments • Dual/Double Sequential Defibrillation • ECMO/ECLS-A New Hope? 3 1 1/24/2018 A Standard Scenario • 55 YOF collapses at stop light, and her car rolls into the car in front of her. Airbags do not deploy, and bystanders find her slumped over the steering wheel and pulseless. • First responder/bystanders start CPR, and deliver three AED shocks prior to EMS arrival. • On EMS arrival, a fourth shock is delivered, an IO and alternative airway placed. Automated CPR started. • Epinephrine 2 mg (total) and Amiodarone 300 mg given, and the patient remains in VF. • Patient downtime is now ~25 minutes, and repeat evaluation reveals persistent VF. 4 What are your options • Continue CPR for a total of 30 minutes with recurrent defibrillations, additional epinephrine, bicarbonate and Amiodarone? • “Load and Go” to the local hospital with CPR enroute and continuing the resuscitation? • “Load and Go” to the local CCL (Cardiac Cath Lab) hospital with CPR enroute and continuing the resuscitation, with possible PCI with ongoing CPR? • Call HEMS unit for transfer to CCL hospital, but can they continue effective CPR in the helicopter? • “Load and Go” to a ECMO/ECLS center with CPR enroute and continuing the resuscitation, on the basis they can accept the patient and continue resuscitation? 5 Defining the problem: What is recurrent versus refractory VF (RVF)? • Recurrent VF is a rhythm that terminates with cardioversion, but then recurs rapidly. -

Arterial Switch Operation Surgery Surgical Solutions to Complex Problems
Pediatric Cardiovascular Arterial Switch Operation Surgery Surgical Solutions to Complex Problems Tom R. Karl, MS, MD The arterial switch operation is appropriate treatment for most forms of transposition of Andrew Cochrane, FRACS the great arteries. In this review we analyze indications, techniques, and outcome for Christian P.R. Brizard, MD various subsets of patients with transposition of the great arteries, including those with an intact septum beyond 21 days of age, intramural coronary arteries, aortic arch ob- struction, the Taussig-Bing anomaly, discordant (corrected) transposition, transposition of the great arteries with left ventricular outflow tract obstruction, and univentricular hearts with transposition of the great arteries and subaortic stenosis. (Tex Heart Inst J 1997;24:322-33) T ransposition of the great arteries (TGA) is a prototypical lesion for pediat- ric cardiac surgeons, a lethal malformation that can often be converted (with a single operation) to a nearly normal heart. The arterial switch operation (ASO) has evolved to become the treatment of choice for most forms of TGA, and success with this operation has become a standard by which pediatric cardiac surgical units are judged. This is appropriate, because without expertise in neonatal anesthetic management, perfusion, intensive care, cardiology, and surgery, consistently good results are impossible to achieve. Surgical Anatomy of TGA In the broad sense, the term "TGA" describes any heart with a discordant ven- triculoatrial (VA) connection (aorta from right ventricle, pulmonary artery from left ventricle). The anatomic diagnosis is further defined by the intracardiac fea- tures. Most frequently, TGA is used to describe the solitus/concordant/discordant heart. -

The Arterial Switch Operation for Transposition of the Great Arteries
The Arterial Switch Operation for Transposition of the Great Arteries Richard A. Jonas Transposition of the great arteries, together with tetral- Ijased on the fact that (luring fetal life hoth ventricles ogy of Fallot, is one of the most common congenital are exposed to the same pressure load. Thus, the left cardiac anomalies resulting in cyanosis. It is hardly ventricle is prepared at birth. However, hecause pulnio- surprising, therefore, that shortly after the introduc- nary resistance falls ra1)idlj within 6 to 8 weeks ancl tion of cardiopulmonary hypass in the early 195Os, perhaps as quicltly as within 2 to 4 weeks, the left attempts were made at anatomical correction of transpo- ventricle is no longer prepared. Iiitroduction of an sition, ie, the arterial switch procedure. These early essentially elective complex neonatal operation in 1983 attempts were uniformly unsuccessful for a number of initially met with consitlerahle resistance, particularly reasons : 1)ecause hy this time the surgical mortality for the atrial 1. Microvascular techniques were not adequately level procedures had reached very low levels. However, developed for delicate coronary artery transfer. a prospectivr study conducted Ijy the Congenital Heart 2. Pulmonary vascular disease is common in transpo- Surgeons Society‘ showed that the overall survival of an sition beyond the first year of life. entire population of patients with transposition en- 3. Surgeons failed to appreciate that in the presence rolled in the neonatal period was superior with the of an intact ventricular septum, the left ventricle was neonatal arterial switch approach because of the previ- inadequately prepared to acntely take over the pressure ously uncaptured deaths that occurred before atrial load of the systemic circulation. -

Minimally Invasive Aortic Valve Replacement: 12-Year Single Center Experience
Featured Article Minimally invasive aortic valve replacement: 12-year single center experience Daniyar Gilmanov, Marco Solinas, Pier Andrea Farneti, Alfredo Giuseppe Cerillo, Enkel Kallushi, Filippo Santarelli, Mattia Glauber Department of Adult Cardiac Surgery, Gabriele Monasterio Tuscany Foundation, G. Pasquinucci Heart hospital, Massa, MS 54100, Italy Correspondence to: Daniyar Sh. Gilmanov. Fellow in Cardiac Surgery – Department of Adult Cardiac Surgery, Gabriele Monasterio Foundation, G. Pasquinucci Heart Hospital, 305, Via Aurelia Sud, loc. Montepepe, Massa, MS 54100, Italy. Email: [email protected]. Background: This study reports the single center experience on minimally invasive aortic valve replacement (MIAVR), performed through a right anterior minithoracotomy or ministernotomy (MS). Methods: Eight hundred and fifty-three patients, who underwent MIAVR from 2002 to 2014, were retrospectively analyzed. Survival was evaluated using the Kaplan-Meier method. The Cox multivariable proportional hazards regression model was developed to identify independent predictors of follow-up mortality. Results: Median age was 73.8, and 405 (47.5%) of patients were female. The overall 30-day mortality was 1.9%. Four hundred and forty-three (51.9%) and 368 (43.1%) patients received biological and sutureless prostheses, respectively. Median cardiopulmonary bypass time and aortic cross-clamping time were 108 and 75 minutes, respectively. Nineteen (2.2%) cases required conversion to full median sternotomy. Thirty- seven (4.3%) patients required re-exploration for bleeding. Perioperative stroke occurred in 15 (1.8%) patients, while transient ischemic attack occurred postoperative in 11 (1.3%). New onset atrial fibrillation was reported for 243 (28.5%) patients. After a median follow-up of 29.1 months (2,676.0 patient-years), survival rates at 1 and 5 years were 96%±1% and 80%±3%, respectively. -

Basic Rhythm Recognition
Electrocardiographic Interpretation Basic Rhythm Recognition William Brady, MD Department of Emergency Medicine Cardiac Rhythms Anatomy of a Rhythm Strip A Review of the Electrical System Intrinsic Pacemakers Cells These cells have property known as “Automaticity”— means they can spontaneously depolarize. Sinus Node Primary pacemaker Fires at a rate of 60-100 bpm AV Junction Fires at a rate of 40-60 bpm Ventricular (Purkinje Fibers) Less than 40 bpm What’s Normal P Wave Atrial Depolarization PR Interval (Normal 0.12-0.20) Beginning of the P to onset of QRS QRS Ventricular Depolarization QRS Interval (Normal <0.10) Period (or length of time) it takes for the ventricles to depolarize The Key to Success… …A systematic approach! Rate Rhythm P Waves PR Interval P and QRS Correlation QRS Rate Pacemaker A rather ill patient……… Very apparent inferolateral STEMI……with less apparent complete heart block RATE . Fast vs Slow . QRS Width Narrow QRS Wide QRS Narrow QRS Wide QRS Tachycardia Tachycardia Bradycardia Bradycardia Regular Irregular Regular Irregular Sinus Brady Idioventricular A-Fib / Flutter Bradycardia w/ BBB Sinus Tach A-Fib VT PVT Junctional 2 AVB / II PSVT A-Flutter SVT aberrant A-Fib 1 AVB 3 AVB A-Flutter MAT 2 AVB / I or II PAT PAT 3 AVB ST PAC / PVC Stability Hypotension / hypoperfusion Altered mental status Chest pain – Coronary ischemic Dyspnea – Pulmonary edema Sinus Rhythm Sinus Rhythm P Wave PR Interval QRS Rate Rhythm Pacemaker Comment . Before . Constant, . Rate 60-100 . Regular . SA Node Upright in each QRS regular . Interval =/< leads I, II, . Look . Interval .12- .10 & III alike .20 Conduction Image reference: Cardionetics/ http://www.cardionetics.com/docs/healthcr/ecg/arrhy/0100_bd.htm Sinus Pause A delay of activation within the atria for a period between 1.7 and 3 seconds A palpitation is likely to be felt by the patient as the sinus beat following the pause may be a heavy beat. -

Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803
Department of Health & CMS Manual System Human Services (DHHS) Pub 100-04 Medicare Claims Processing Centers for Medicare & Medicaid Services (CMS) Transmittal 3054 Date: August 29, 2014 Change Request 8803 SUBJECT: Ventricular Assist Devices for Bridge-to-Transplant and Destination Therapy I. SUMMARY OF CHANGES: This Change Request (CR) is effective for claims with dates of service on and after October 30, 2013; contractors shall pay claims for Ventricular Assist Devices as destination therapy using the criteria in Pub. 100-03, part 1, section 20.9.1, and Pub. 100-04, Chapter 32, sec. 320. EFFECTIVE DATE: October 30, 2013 *Unless otherwise specified, the effective date is the date of service. IMPLEMENTATION DATE: September 30, 2014 Disclaimer for manual changes only: The revision date and transmittal number apply only to red italicized material. Any other material was previously published and remains unchanged. However, if this revision contains a table of contents, you will receive the new/revised information only, and not the entire table of contents. II. CHANGES IN MANUAL INSTRUCTIONS: (N/A if manual is not updated) R=REVISED, N=NEW, D=DELETED-Only One Per Row. R/N/D CHAPTER / SECTION / SUBSECTION / TITLE D 3/90.2.1/Artifiical Hearts and Related Devices R 32/Table of Contents N 32/320/Artificial Hearts and Related Devices N 32/320.1/Coding Requirements for Furnished Before May 1, 2008 N 32/320.2/Coding Requirements for Furnished After May 1, 2008 N 32/320.3/ Ventricular Assist Devices N 32/320.3.1/Postcardiotomy N 32/320.3.2/Bridge-To -Transplantation (BTT) N 32/320.3.3/Destination Therapy (DT) N 32/320.3.4/ Other N 32/320.4/ Replacement Accessories and Supplies for External Ventricular Assist Devices or Any Ventricular Assist Device (VAD) III.