Calcium Gluconate Injection
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Management of Poisoning
MOH CLINICAL PRACTICE GUIDELINES December/2011 Management of Poisoning Health Ministry of Sciences Chapter of Emergency College of College of Family Manpower Authority Physicians Physicians, Physicians Academy of Medicine, Singapore Singapore Singapore Singapore Medical Pharmaceutical Society Society for Emergency Toxicology Singapore Paediatric Association of Singapore Medicine in Singapore Society (Singapore) Society Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Principles of management of acute poisoning – resuscitating the poisoned patient GPP In a critically poisoned patient, measures beyond standard resuscitative protocol like those listed above need to be implemented and a specialist experienced in poisoning management should be consulted (pg 55). GPP D Prolonged resuscitation should be attempted in drug-induced cardiac arrest (pg 55). Grade D, Level 3 1 C Titrated doses of naloxone, together with bag-valve-mask ventilation, should be administered for suspected opioid-induced coma, prior to intubation for respiratory insuffi ciency (pg 56). Grade C, Level 2+ D In bradycardia due to calcium channel or beta-blocker toxicity that is refractory to conventional vasopressor therapy, intravenous calcium, glucagon or insulin should be used (pg 57). Grade D, Level 3 B Patients with actual or potential life threatening cardiac arrhythmia, hyperkalaemia or rapidly progressive toxicity from digoxin poisoning should be treated with digoxin-specifi c antibodies (pg 57). Grade B, Level 2++ B Titrated doses of benzodiazepine should be given in hyperadrenergic- induced tachycardia states resulting from poisoning (pg 57). Grade B, Level 1+ D Non-selective beta-blockers, like propranolol, should be avoided in stimulant toxicity as unopposed alpha agonism may worsen accompanying hypertension (pg 57). -

GHS Calcium Lactate Gluconate MSDS.Pdf
Safety Data Sheet (Calcium Lactate Gluconate) DATE PREPARED: 7/9/2015 Section 1. Product and Company Identification Product Name Calcium Lactate Gluconate CAS Number 11116-97-5 Parchem - fine & specialty chemicals EMERGENCY RESPONSE NUMBER 415 Huguenot Street CHEMTEL New Rochelle, NY 10801 Toll Free US & Canada: 1 (800) 255-3924 (914) 654-6800 (914) 654-6899 All other Origins: 1 (813) 248-0585 parchem.com [email protected] Collect Calls Accepted Section 2. Hazards Identification Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended: This preparation does not meet the criteria for classification according to Directive 1999/45/EC as amended. Classification according to Regulation (EC) No 1272/2008 as amended: This mixture does not meet the criteria for classification according to Regulation (EC) 1272/2008 as amended. Hazard and precautionary statements Hazard statements: The substance does not meet the criteria for classification. Precautionary statements: Not available. Appearance & Odor: White powder with no odor. Fire & Explosion Hazards Potential for dust explosion may exist. This product is not defined as flammable or combustible. However, the product may decompose under fire conditions to produce toxic oxides of carbon. Depending upon conditions, dusts may be sensitive to static discharge. Avoid possibility of dry powder and friction causing static electricity in presence of flammables (See NFPA-77, Chpt. 6) Primary Route of Exposure: Skin and eye contact are the primary routes of exposure to this product. Inhalation Acute Exposure: Inhalation of dust may cause mild irritation. Skin Contact - Acute: Skin contact is not expected to cause irritation. -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -

Calcium Gluconate
CALCIUM GLUCONATE CLASSIFICATION Minerals and electrolytes TRADE NAME(S) Calcium Gluconate 10% (for IV use) Calcium Gluconate gel 2.5% (for topical use) DESIRED EFFECTS Lower potassium levels; pain relief and neutralizing fluoride ion MECHANISM OF ACTION Calcium is the primary component of skeletal tissue. Bone serves as a calcium depot and as a reservoir for electrolytes and buffers. INDICATIONS Suspected hyperkalemia in adult PEA/Asystole Antidote for calcium channel blocker overdose and magnesium sulfate toxicity Gel is used for hydrofluoric acid burns Suspected hyperkalemia with adult crush injury or peaked T-waves on EKG CONTRAINDICATIONS Should not be given to patients with digitalis toxicity Should be used with caution in patients with dehydration ADVERSE REACTIONS When given too rapidly or to someone on digitalis, can cause sudden death from ventricular fibrillation May cause mild to severe IV site irritation SPECIAL CONSIDERATIONS Must either use a different IV line or flush line with copious normal saline if being given with sodium bicarbonate. When used on hydroflouric acid burns, relief of pain is the only indication of treatment efficacy. Therefore, use of analgesic agents is not recommended. DOSING REGIMEN Suspected hyperkalemia in adult PEA/asystole or adult crush injury, or evidence of EKG changes (Ex. peaked T-waves) o Calcium gluconate 10% 15-30 ml IV/IO over 2-5 minutes KNOWN calcium channel blocker overdose - administer 3 grams IV/IO may repeat dose in 10 minutes if no effect. Hydrofluoric acid burns apply calcium gluconate gel 2.5% every 15 minutes to burned area and massage continuously until pain disappears. -
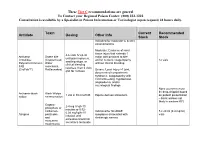
Antidote Stocking Tier C
These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Toxin Current Recommended Antidote Dosing Other Info Stock Stock Indicated for moderate to severe envenomations Moderate: Evidence of local tissue injury that extends 1 4-6 vials IV q2-4h Antivenin Snake bite major joint proximal to bite until pain improves, Crotalidae (Copperhead, and/or numeric coagulopathy 12 vials swelling stops, or Polyvalent Immune Water without clinical bleeding clinical bleeding FAB moccasins, resolves, then 2 vials (CroFab™) Rattlesnakes) Severe: Local injury >1 joint, q6h for 3 doses documented compartment syndrome, coagulopathy with clinical bleeding, hypotension, angioedema, and/or neurological findings None (currently must be drop-shipped based Antivenin-black Black Widow 1 vial in 50 ml of NS Equine derived antivenom on patient presentation widow envenomation – black widows not likely in western KY) Organo- 2-4 mg IV q5-10 phosphate or minutes or 0.02- carbamate Indicated for SLUDGE 5 x 20 ml (0.4 mg/ml) 0.08 mg/kg/hr IV Atropine pesticides symptoms associated with vials infusion until and cholinergic excess excessive bronchial muscarine secretions terminate mushrooms These Tier C recommendations are general To Contact your Regional Poison Center: (800) 222-1222 Consultation is available by a Specialist in Poison Information or Toxicologist (upon request) 24 hours daily. Antidote Current Recommended Toxin -

Calcium Gluconate Injection Contains 95 Mg/Ml Calcium Gluconate Monohydrate and 3.0 Mg/Ml Calcium Saccharate
Calcium Gluconate Injection Contains 95 mg/mL Calcium Gluconate Monohydrate and 3.0 mg/mL Calcium Saccharate Consumer Medicine Information What is in this leaflet Before you are given You should not be given Calcium Gluconate Injection if you have been Calcium Gluconate bed ridden for a long time causing This leaflet answers some common the loss of calcium from the bones. questions about Calcium Gluconate Injection Injection. It does not contain all the You should not be given Calcium available information. It does not take Gluconate Injection if you are being the place of talking to your doctor. When you must not be given treated with certain heart drugs such it as digoxin and digitalis. All medicines have risks and benefits. Your doctor has weighed the risks of You should not be given Calcium You should not be given Calcium you being given Calcium Gluconate Gluconate Injection if you have an Gluconate Injection if the solution is Injection against the benefits they allergy to: discoloured, cloudy, turbid, or a expect it will have for you. • any medicine containing calcium precipitate is present. The solution is gluconate normally a clear, colourless liquid. If you have any concerns about being given this medicine, ask your • any of the ingredients listed at the You should not be given Calcium doctor. end of this leaflet. Gluconate Injection if when diluted with another solution it causes the Some of the symptoms of an allergic Keep this leaflet. solution to precipitate, become You may need to read it again. reaction may include: cloudy, turbid, discolour, or particles • shortness of breath are visible. -

Obstetric Safety
Obstetric Safety DIAGNOSIS OF LABOUR FIRST STAGE . Latent phase . Cervix less than 4 cm dilated . Active phase . Cervix between 4 cm and 10 cm dilated SECOND STAGE . Early phase (non-expulsive) . Cervix fully dilated (10 cm) . Fetal descent continues . Patient has no urge to push . Late phase (expulsive) . Presenting part of fetus reaches the pelvic floor and the patient has the urge to push . Typically lasts less than 1 hour in primigravida women and less than 30 minutes in multigravida women Carry .out Dedicated vaginal examinations equipment at leastfor procedures once every 4 hours in the first stage of labour. andEquipment plot the findings to monitor on the patients, partograph. as required for the procedure . Drugs and other consumables for routine and emergency use The partograph is very helpful in monitoring the progress of labour and in the early detection of abnormal labour patterns. WHO/HPW/CPR 2004 reformatted 2012 1 DIAGNOSIS OF VAGINAL BLEEDING IN EARLY PREGNANCY Typical Symptoms and Probable Occasional Symptoms and Signs Signs Diagnosis . Light* bleeding . Cramping/lower abdominal pain Threatened . Closed cervix . Uterus softer than normal abortion . Uterus corresponds to dates . Light bleeding . Abdominal pain . Fainting . Closed cervix . Tender adnexal mass Ectopic . Uterus slightly larger than . Amenorrhea pregnancy normal . Cervical motion tenderness . Uterus softer than normal . Light bleeding . Light cramping/lower abdominal pain . Closed cervix Complete . History of expulsion of products of . Uterus smaller than dates abortion conception . Uterus softer than normal . Cramping/lower abdominal pain . Heavy** bleeding . Tender uterus Inevitable . Dilated cervix . No expulsion of products of abortion . Uterus corresponds to dates conception . Heavy bleeding . -

The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
The Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island Poison Potential Antidote Acetaminophen n-Acetylcysteine [Mucomyst®] Anticholinergics Physostigmine [Antilirium®] Benzodiazepines Flumazenil [Romazicon®] Beta-adrenergic blockers Glucagon Botulinum toxin Trivalent ABE botulinum antitoxin Calcium chloride or calcium gluconate Calcium channel blockers Hyperinsulinemia-euglycemia (HIE) therapy Atropine Carbamates Pralidoxime (2-PAM) [Protopam®] Carbon monoxide Oxygen; Hyperbaric oxygen (HBO) Clonidine Naloxone [Narcan®] Cyanide Cyanide Kit (Amyl/sodium nitrite, sodium thiosulfate) Digoxin (Cardiac glycosides) Digoxin Immune FAB Ovine [Digibind®, Digifab®] Epi Pen (Epinephrine SQ) Phentolamine [Regitine®] Ethanol Ethylene glycol 4-Methylpyrazole (Fomepizole) [Antizol®] Fluoride Calcium chloride or calcium gluconate Heparins Protamine Hydrofluoric acid Calcium chloride or calcium gluconate Hydrogen sulfide Oxygen; Hyperbaric oxygen (HBO); Sodium nitrite Iodine Starch Isoniazid Pyridoxine (Vitamin B6 ) METALS Dimercaprol [BAL] Arsenic Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Bismuth Dimercaprol [BAL] Copper D-Penicillamine [Cuprimine®] Gold Dimercaprol [BAL] Iron Deferoxamine [Desferal®] Dimercaprol [BAL] Edetate calcium disodium (Calcium EDTA) Lead Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] D-Penicillamine [Cuprimine®] Dimercaprol [BAL] Mercury Dimercaptosuccinic acid (DMSA, succimer) [Chemet®] Ethanol Methanol 4-Methylpyrazole (Fomepizole) [Antizol®] Methemoglobinemic agents Methylene -

Man with Syncope Emily Lai MD, PGY-2 and Anita Mehrotra MD, PGY-1
Pulse: EKG Man with Syncope Emily Lai MD, PGY-2 and Anita Mehrotra MD, PGY-1 Mr. K, a 57 year-old Caucasian male with a history of atorvastatin, furosemide, aspirin and niacin. He had no coronary artery disease, dilated ischemic cardiomy- known drug allergies. Mr. K was a retired train opathy, ventricular fibrillation arrest status post conductor. He smoked half of a pack of cigarettes a day implantable cardioverter defibrillator (ICD) placement and had an 80 pack-year smoking history. He had a was admitted to telemetry for two syncopal episodes. The history of alcohol, cocaine, and amphetamine abuse, but patient described multiple episodes of dizziness with he quit these drugs 15 years prior. He denied any history fatigue over the week prior to admission, the last two of intravenous drug use. episodes resulting in loss of consciousness with bladder Physical examination on admission was significant for incontinence. He was home alone when these episodes elevated jugular venous pressure, a grade II/VI systolic occurred and was unsure of how long he had been ejection murmur over the left sternal border, diffuse unconscious. Mr. K was not aware of any recent firing of wheezes on lung exam, and 2+ lower extremity edema. his ICD. He denied any shortness of breath, chest pain, Laboratory results showed a B-type natriuretic peptide diaphoresis, recent fevers or chills. level of 662 pg/ml, a creatinine of 1.0 mg/dL, and a His medical history was significant for hypertension, digoxin level of 1.6 ng/mL. Chest X-ray showed coronary artery disease status post two vessel coronary moderate pulmonary edema. -

LCCH Emergency Resuscitation Infusion Guide
LCCH Emergency Resuscitation Infusion Guide Drug & Presentation Reconstitution & Final drug Dosage range Route & Administration Comments Dilution concentration Acetylcysteine inj See N-Acetylcysteine (NAC) adrenaline 1:1000 1mg in 50mL 0.02mg/mL 0.01 to 2 microg/kg/min IV infusion Central Line with 0.9% NaCl / 5% glucose Incompatible with Sodium Bicarbonate alPROStadil (PGE1) 100microg in 50mL 2microg/mL 0.01 to 400 nanog/kg/min IV infusion Stored in fridge (prostaglandin) with 0.9% NaCl (equals 2000 nanog/mL) May cause apnoea amINOPHYLLIne inj LOAD 250mg in 50mL 5mg/mL Asthma LOAD: IV infusion of exact dose Alkaline (ph 8.6-9) with 5% Glucose / 5 mg/kg over 30 to 60 mins 0.9% NaCl amINOPHYLLIne INFUS 250mg in 50mL 5mg/mL 0.5 to 1.5 mg/kg/hr IV infusion of exact dose Alkaline (ph 8.6-9) with 5% Glucose / Usually 10mg/kg over 30- over 60 mins NOTE: Usually initiated at PICU 0.9% NaCl 60mins discretion amIODARone CVL(<45kg) 300mg in 50mL 6mg/mL 5 to 15 microg/kg/min IV infusion Incompatible with sodium chloride with 5% glucose solutions amIODARone CVL(>45kg) 300mg in 50mL 6mg/mL 0.01 to 37 mg/hr IV infusion Monitor ECG and blood pressure with 5% glucose amIODARone PERIPH 100mg in 50mL 2mg/mL 5 to 15 microg/kg/min IV infusion Incompatible with sodium chloride (<45kg) with 5% glucose solutions Monitor ECG and blood pressure amIODARone PERIPH 900mg in 450ml 2mg/mL 0.01 to 37 mg/hr IV infusion (>45kg) with 5% glucose calcium CHLORide 6.8mmol in 10mL 0.68mmol/mL 0.01 to 0.61 mmol/kg/hr IV infusion Central Line only (CVL only) with 5% glucose / 0.9% NaCl LCCH Emergency Resuscitation Infusion Guide - 1 - Version 3, 22 March 2015. -

10D) Drugs (Dosage, Administration, Precautions and Monitoring
INSTRUCTIONS FOR ONCOLOGY WARD (10D) DRUGS (DOSAGE, ADMINISTRATION, PRECAUTIONS AND MONITORING) PREPEARED BY CLINICAL PHARMACIST: ESHRAQ ALABWEENY THE SUPERVISOR OF DRUG INFORMATION CENTER / JUST 1 . Calcium Gluconate 10 % ( 10 ml) Dosage: Hypocalcemia: IV: Mild (ionized calcium: [1 to 1.2 mmol/L]): 1 to 2 g over 2 hours; asymptomatic patients may be given oral calcium. Moderate to severe (without seizure or tetany; ionized calcium: [<1 mmol/L]): 4 g over 4 hours. Severe symptomatic (eg, seizure, tetany): 1 to 2 g over 10 minutes; repeat every 60 minutes until symptoms resolve. Note: Repeat ionized calcium measurement 6 to 10 hours after completion of administration. Check for hypomagnesemia and correct if present. Consider continuous infusion if hypocalcemia is likely to recur due to ongoing losses. Continuous infusion: 5 to 20 mg/kg/hour; in patients with hypoparathyroidism, oral calcium and active vitamin D (ie, calcitriol) with or without ergocalciferol or cholecalciferol should be initiated as soon as is practical; IV calcium is generally tapered slowly while oral therapy is adjusted . Hypocalcemia induced by citrate-based replacement fluid during continuous renal replacement therapy (CRRT): IV (administered via return line): Note: Prior to initiation of CRRT, check ionized calcium and administer calcium gluconate if (<1 mmol/L) until (>1 mmol/L). During CRRT, a continuous infusion sliding scale may be initiated (may use calcium gluconate 20 gram/1,000 mL NS or D5W solution). The following schema has been employed : If ionized calcium is (<0.9 mmol/L): Notify nephrology. If ionized calcium is (0.9 to 1 mmol/L): 1.4 g/hour. -
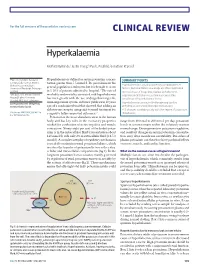
Clinical Review Hyperkalaemia
For the full versions of these articles see bmj.com CLINICAL REVIEW Hyperkalaemia Moffat J Nyirenda,1 Justin I Tang,1 Paul L Padfield,2 Jonathan R Seckl1 1Endocrinology Unit, Centre for Hyperkalaemia is defined as serum potassium concen- SUMMARY POINTS Cardiovascular Science, Queen’s tration greater than 5.5 mmol/l. Its prevalence in the Medical Research Institute, Hyperkalaemia is usually caused by a combination of University of Edinburgh, Edinburgh general population is unknown, but it is thought to occur factors, but renal failure and drugs are often implicated 1 EH16 4TJ in 1-10% of patients admitted to hospital. The rate of Increased use of drugs that interact with the renin- 2Metabolic Unit, Western General morbidity and mortality associated with hyperkalaemia angiotensin-aldosterone system has caused the Hospital, Lothian University has risen greatly with the use of drugs that target the prevalence of hyperkalaemia to rise Hospitals NHS Trust, Edinburgh renin-angiotensin system, and since publication 10 years Correspondence to: M Nyirenda Hyperkalaemia can cause life threatening cardiac [email protected] ago of a randomised trial that showed that adding an arrhythmias and should be urgently managed aldosterone receptor antagonist to usual treatment for ECG changes correlate poorly with the degree of potassium Cite this as: BMJ 2009;339:b4114 congestive failure improved outcomes.2-5 disturbance doi: 10.1136/bmj.b4114 Potassium is the most abundant cation in the human body and has key roles in the excitatory properties range from 40 mmol to 200 mmol per day, potassium needed for conduction of nerve impulses and muscle levels in serum remain within the relatively narrow contraction.