Pediatric Guidelines for IV Medication Administration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surfen, a Small Molecule Antagonist of Heparan Sulfate
Surfen, a small molecule antagonist of heparan sulfate Manuela Schuksz*†, Mark M. Fuster‡, Jillian R. Brown§, Brett E. Crawford§, David P. Ditto¶, Roger Lawrence*, Charles A. Glass§, Lianchun Wang*, Yitzhak Torʈ, and Jeffrey D. Esko*,** *Department of Cellular and Molecular Medicine, Glycobiology Research and Training Center, †Biomedical Sciences Graduate Program, ‡Department of Medicine, Division of Pulmonary and Critical Care Medicine and Veteran’s Administration San Diego Medical Center, ¶Moores Cancer Center, and ʈDepartment of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA 92093; and §Zacharon Pharmaceuticals, Inc, 505 Coast Blvd, South, La Jolla, CA 92037 Communicated by Carolyn R. Bertozzi, University of California, Berkeley, CA, June 18, 2008 (received for review May 26, 2007) In a search for small molecule antagonists of heparan sulfate, Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) was first we examined the activity of bis-2-methyl-4-amino-quinolyl-6- described in 1938 as an excipient for the production of depot carbamide, also known as surfen. Fluorescence-based titrations insulin (16). Subsequent studies have shown that surfen can indicated that surfen bound to glycosaminoglycans, and the extent block C5a receptor binding (17) and lethal factor (LF) produced of binding increased according to charge density in the order by anthrax (18). It was also reported to have modest heparin- heparin > dermatan sulfate > heparan sulfate > chondroitin neutralizing effects in an oral feeding experiments in rats (19), sulfate. All charged groups in heparin (N-sulfates, O-sulfates, and but to our knowledge, no further studies involving heparin have carboxyl groups) contributed to binding, consistent with the idea been conducted, and its effects on HS are completely unknown. -

Approved Livestock Drug Registrations
Livestock Drug Labels‐Approved and Provisional Status Firm: Product Name, Brand or Trademark Product Category Provisional ADEPTUS NUTRITION INC NIMBLE MEGA NUTRIENT (S&E RECEIVED) Physiological (Structure/Function) Provisional ADEPTUS NUTRITION INC NIMBLE ULTRA (S & E Received) Physiological (Structure/Function) Provisional ADEPTUS NUTRITION INC NIMBLE SUPREME (S & E Received) Physiological (Structure/Function) Approved ADEPTUS NUTRITION INC ADEPTUS WOUND AND SKIN SPRAY Topical Approved AFS DISTRIBUTING DURASOLE Topical Approved AGRI LABORATORIES LTD FERRRODEX 100 Injectable Approved AGRI LABORATORIES LTD VITAMIN E‐300 Injectable Approved AGRI LABORATORIES LTD PROPYLENE GLYCOL Physiological (Structure/Function) Approved AGRI LABORATORIES LTD IODINE WOUND SPRAY Topical Approved AGRI LABORATORIES LTD AGRI‐MECTIN (IVERMECTIN) INJECTION FOR CATTLE AND SWINE (RD) Restricted Drug‐/‐Wormer Approved AGRI LABORATORIES LTD AGRI‐MECTIN (IVERMECTIN) POUR‐ON FOR CATTLE (RD) Restricted Drug‐/‐Wormer Approved AGRI LABORATORIES LTD DEXTROSE 50% Injectable Approved AGRI LABORATORIES LTD PROHIBIT (LEVAMISOL HYDROCHLORIDE) SOLUBLE DRENCH POWDER (RD) Restricted Drug‐/‐Wormer Approved AGRI LABORATORIES LTD KAO‐PEC ANTI‐DIARRHEAL LIQUID Physiological (Structure/Function) Approved AGRI LABORATORIES LTD AGRIMYCIN 200 (OXYTETRACYCLINE) (CA RX RD) Restricted Drug‐/‐Injectable Approved AGRI LABORATORIES LTD VITAMIN E‐AD 300 INJECTABLE TOCOPHEROL WITH A+D Injectable Approved AGRI LABORATORIES LTD VITAMIN A D INJECTION Injectable Approved AGRI LABORATORIES LTD FORTIFIED -

Management of Poisoning
MOH CLINICAL PRACTICE GUIDELINES December/2011 Management of Poisoning Health Ministry of Sciences Chapter of Emergency College of College of Family Manpower Authority Physicians Physicians, Physicians Academy of Medicine, Singapore Singapore Singapore Singapore Medical Pharmaceutical Society Society for Emergency Toxicology Singapore Paediatric Association of Singapore Medicine in Singapore Society (Singapore) Society Executive summary of recommendations Details of recommendations can be found in the main text at the pages indicated. Principles of management of acute poisoning – resuscitating the poisoned patient GPP In a critically poisoned patient, measures beyond standard resuscitative protocol like those listed above need to be implemented and a specialist experienced in poisoning management should be consulted (pg 55). GPP D Prolonged resuscitation should be attempted in drug-induced cardiac arrest (pg 55). Grade D, Level 3 1 C Titrated doses of naloxone, together with bag-valve-mask ventilation, should be administered for suspected opioid-induced coma, prior to intubation for respiratory insuffi ciency (pg 56). Grade C, Level 2+ D In bradycardia due to calcium channel or beta-blocker toxicity that is refractory to conventional vasopressor therapy, intravenous calcium, glucagon or insulin should be used (pg 57). Grade D, Level 3 B Patients with actual or potential life threatening cardiac arrhythmia, hyperkalaemia or rapidly progressive toxicity from digoxin poisoning should be treated with digoxin-specifi c antibodies (pg 57). Grade B, Level 2++ B Titrated doses of benzodiazepine should be given in hyperadrenergic- induced tachycardia states resulting from poisoning (pg 57). Grade B, Level 1+ D Non-selective beta-blockers, like propranolol, should be avoided in stimulant toxicity as unopposed alpha agonism may worsen accompanying hypertension (pg 57). -

Association of Hypertensive Status and Its Drug Treatment with Lipid and Haemostatic Factors in Middle-Aged Men: the PRIME Study
Journal of Human Hypertension (2000) 14, 511–518 2000 Macmillan Publishers Ltd All rights reserved 0950-9240/00 $15.00 www.nature.com/jhh ORIGINAL ARTICLE Association of hypertensive status and its drug treatment with lipid and haemostatic factors in middle-aged men: the PRIME Study P Marques-Vidal1, M Montaye2, B Haas3, A Bingham4, A Evans5, I Juhan-Vague6, J Ferrie`res1, G Luc2, P Amouyel2, D Arveiler3, D McMaster5, JB Ruidavets1, J-M Bard2, PY Scarabin4 and P Ducimetie`re4 1INSERM U518, Faculte´ de Me´decine Purpan, Toulouse, France; 2MONICA-Lille, Institut Pasteur de Lille, Lille, France; 3MONICA-Strasbourg, Laboratoire d’Epide´miologie et de Sante´ Publique, Strasbourg, France; 4INSERM U258, Hoˆ pital Broussais, Paris, France; 5Belfast-MONICA, Department of Epidemiology, The Queen’s University of Belfast, UK; 6Laboratory of Haematology, La Timone Hospital, Marseille, France Aims: To assess the association of hypertensive status this effect remained after multivariate adjustment. Cal- and antihypertensive drug treatment with lipid and hae- cium channel blockers decreased total cholesterol and mostatic levels in middle-aged men. apoproteins A-I and B; those differences remained sig- Methods and results: Hypertensive status, antihyperten- nificant after multivariate adjustment. ACE inhibitors sive drug treatment, total and high-density lipoprotein decreased total cholesterol, triglycerides, apoprotein B (HDL) cholesterol, triglyceride, apoproteins A-I and B, and LpE:B; and this effect remained after multivariate lipoparticles LpA-I, -

Tube Feeding Using the Bolus Method | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Tube Feeding Using the Bolus Method This information will help teach you how to use the bolus method to feed yourself and take your medications through your percutaneous endoscopic gastrostomy (PEG), gastrostomy tube (GT), or nasogastric tube (NGT). About Tube Feeding Tube feeding is when you get your nutrients through a feeding tube if you aren’t able to get enough through eating and drinking, or if you can’t swallow safely. Nutrients provide energy and help you heal. The bolus method is a type of feeding where a syringe is used to send formula through your feeding tube. The syringe you’ll use is called a catheter syringe. A catheter syringe doesn’t have a needle. It has a hole with a plunger in it. You draw up formula through the hole in the syringe then push the formula into your feeding tube with the plunger. A bolus refers to 1 “meal” of formula. You may have a feeding tube with a legacy connector or an ENFit connector. In this resource, we’ll show images of both types of connectors. For more information about your feeding tube, including how to manage side effects, read Tube Feeding Troubleshooting Guide. Tube Feeding Using the Bolus Method 1/11 Tube Feeding Guidelines Formula: __________ Total cans per day: ____________________ (8 ounces each) Calories per day: __________ You can choose the times of your feedings, as long as you reach your daily nutritional goals. Write in the times you prefer or your doctor, advanced practice provider (APP), or clinical dietitian nutritionist recommends. -

Outpatient Parenteral Antimicrobial Therapy (OPAT) Self-Administration of Meropenem 500Mg IV Bolus
Outpatient Parenteral Antimicrobial Therapy (OPAT) Self-administration of Meropenem 500mg IV bolus This leaflet is designed to support patients, and nursing staff who are teaching patients to self-administer Meropenem, with the assistance of the OPAT team. Please use this information in conjunction with the ‘Patient Self-Administration IV Therapy Competency Tool’. Through comprehensive individual demonstration, training and assessment, you will be able to: • Minimise the risk of introducing infection into a vein by keeping everything very clean. • Avoid touching the key parts of syringes, needles and extension sets. • Prevent injecting air into a vein by learning to prime syringes and extension sets carefully. What is Meropenem? Meropenem is an antibiotic; it is part of the antibiotic group carbapenem. Meropenem is given intravenously, by multiple doses throughout the day. Meropenem is only available as an injection. Please read the patient information leaflet inside medication box for further information about your medication. What you will need The ward nursing team and the OPAT nurses will ensure that you are happy and safe to administer Meropenem following the procedure below: Source: Outpatient Parenteral Antimicrobial Therapy Service Reference No: 6404-2 Issue date: 22/6/20 Review date: 22/6/23 Page no: 1 Equipment per 1 tray and sani-cloth detergent wipes dose: 1 pair sterile gloves and non-sterile gloves 3 10ml syringes 3 red needles 2 clinell wipes 2% 3 red bungs and sharps bin • 10ml ampoules 0.9% sodium chloride (normal saline) x2 Patient dose: 500mg • Meropenem 500mg vial and an ampoule of 10 mls water for injection What to do Remember to check the dose and the expiry date of the drug, diluent and normal saline. -

GHS Calcium Lactate Gluconate MSDS.Pdf
Safety Data Sheet (Calcium Lactate Gluconate) DATE PREPARED: 7/9/2015 Section 1. Product and Company Identification Product Name Calcium Lactate Gluconate CAS Number 11116-97-5 Parchem - fine & specialty chemicals EMERGENCY RESPONSE NUMBER 415 Huguenot Street CHEMTEL New Rochelle, NY 10801 Toll Free US & Canada: 1 (800) 255-3924 (914) 654-6800 (914) 654-6899 All other Origins: 1 (813) 248-0585 parchem.com [email protected] Collect Calls Accepted Section 2. Hazards Identification Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended: This preparation does not meet the criteria for classification according to Directive 1999/45/EC as amended. Classification according to Regulation (EC) No 1272/2008 as amended: This mixture does not meet the criteria for classification according to Regulation (EC) 1272/2008 as amended. Hazard and precautionary statements Hazard statements: The substance does not meet the criteria for classification. Precautionary statements: Not available. Appearance & Odor: White powder with no odor. Fire & Explosion Hazards Potential for dust explosion may exist. This product is not defined as flammable or combustible. However, the product may decompose under fire conditions to produce toxic oxides of carbon. Depending upon conditions, dusts may be sensitive to static discharge. Avoid possibility of dry powder and friction causing static electricity in presence of flammables (See NFPA-77, Chpt. 6) Primary Route of Exposure: Skin and eye contact are the primary routes of exposure to this product. Inhalation Acute Exposure: Inhalation of dust may cause mild irritation. Skin Contact - Acute: Skin contact is not expected to cause irritation. -

Podder™ Resource Guide Omnipod DASH® System
Podder™ Resource Guide Omnipod DASH® System Insulin Delivery That’s Simple, Smart, and Discreet Introduction | TABLE OF CONTENTS Get to Know the Omnipod DASH® System Introduction 04 Advanced Features 30 Welcome 04 Bolus 30 Supply List and Reorder 05 Basal 31 The Pod 06 Food Library 34 The Personal Diabetes Manager 07 Custom Foods 35 Basal/Bolus 10 Presets 36 Your Personal Diabetes Manager Settings 11 Counting Carbohydrates 12 Troubleshooting 39 Sick Day Management 39 Omnipod DASH® System Instructions 14 Hypoglycemia 40 How to Change the Pod 14 Hyperglycemia 42 Activate a New Pod 15 Notifications, Alerts & Alarms 44 Pod Placement/Prep/Tips 20 Blood Glucose Meter Pairing 22 Digital Resources 46 Blood Glucose Meter Syncing 23 Additional Notes 47 Delivering a Bolus 24 Suspend and Resume Insulin Delivery 25 Important Tips and Reminders 26 Additional Notes 29 Contact your local Omnipod® System representative or visit omnipod.com for more information. This Resource Guide is intended to be used in conjunction with your Diabetes Management Plan, input from your healthcare provider, and the Omnipod DASH® Insulin Management System User Guide. Personal Diabetes Manager imagery is for illustrative purposes only and should not be considered suggestions for user settings. Refer to the Omnipod DASH® Insulin Management System User Guide for complete information on how to use the Omnipod DASH® System, and for all related warnings and cautions. The Omnipod DASH® Insulin Management System User Guide is available online at omnipod.com or by calling Customer Care (24 hours/7 days), at 800-591-3455. CAUTION: Consult User Guide. This Resource Guide is for Personal Diabetes Manager model PDM-USA1-D001-MG-USA1. -

Caregiver Guide
INSULIN MANAGEMENT SYSTEM CAREGIVER GUIDE SIMPLE, NONSTOP INSULIN DELIVERY DESIGNED SO KIDS CAN BE KIDS 2 GET TO KNOW OMNIPOD.® WHAT’S DIFFERENT + The Pod . 2 ABOUT THE POD? SIMPLE. + The PDM (Personal Diabetes Manager) . .. 3 ® + How to check blood glucose and deliver a bolus . 4 OmniPod provides up to 3 days of nonstop insulin delivery* so kids with diabetes can run, play, and move, all while staying in control of their insulin . The system is simply 2 parts—the + How to change the Pod . 8 tubeless Pod and the handheld Personal Diabetes Manager (PDM) that your child keeps nearby Pod Placement Options . 10 so you can both wirelessly program insulin delivery . The Pod is waterproof †, lightweight, and Activate a New Pod . 11 discreet, and can be worn anywhere you would give an injection . OmniPod® helps simplify Step 1: Fill the Pod . 11 insulin delivery, so kids can be kids and you can worry less . That’s just part of what makes Step 2: Apply the Pod . 13 people so passionate about the Pod . Step 3: Press Start . 15 ® + How to enter a temporary basal rate . 16 Preparing your child to start on OmniPod . + How to suspend insulin delivery . 18 Whether you’re a school nurse, daycare provider, parent, grandparent, or other secondary + Supplies . 20 caregiver for a child using the OmniPod® Insulin Management System, this guide will lead you through some of the key functions you may need to perform . This guide is intended to be used in conjunction with the child’s Diabetes Management Plan, input from the parents and/or healthcare provider and the OmniPod® Insulin Management Have questions? System User Guide . -

Wednesday May 26, 1999
5±26±99 Vol. 64 No. 101 Wednesday Pages 28333±28712 May 26, 1999 federal register 1 VerDate 06-MAY-99 21:29 May 25, 1999 Jkt 183247 PO 00000 Frm 00001 Fmt 4710 Sfmt 4710 E:\FR\FM\26MYWS.XXX pfrm03 PsN: 26MYWS II Federal Register / Vol. 64, No. 101 / Wednesday, May 26, 1999 The FEDERAL REGISTER is published daily, Monday through SUBSCRIPTIONS AND COPIES Friday, except official holidays, by the Office of the Federal Register, National Archives and Records Administration, PUBLIC Washington, DC 20408, under the Federal Register Act (44 U.S.C. Subscriptions: Ch. 15) and the regulations of the Administrative Committee of Paper or fiche 202±512±1800 the Federal Register (1 CFR Ch. I). The Superintendent of Assistance with public subscriptions 512±1806 Documents, U.S. Government Printing Office, Washington, DC 20402 is the exclusive distributor of the official edition. General online information 202±512±1530; 1±888±293±6498 Single copies/back copies: The Federal Register provides a uniform system for making available to the public regulations and legal notices issued by Paper or fiche 512±1800 Federal agencies. These include Presidential proclamations and Assistance with public single copies 512±1803 Executive Orders, Federal agency documents having general FEDERAL AGENCIES applicability and legal effect, documents required to be published Subscriptions: by act of Congress, and other Federal agency documents of public Paper or fiche 523±5243 interest. Assistance with Federal agency subscriptions 523±5243 Documents are on file for public inspection in the Office of the Federal Register the day before they are published, unless the issuing agency requests earlier filing. -
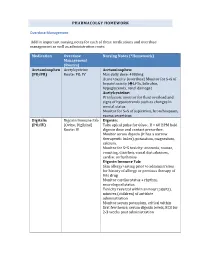
PHARMACOLGY HOMEWORK Overdose Management Add In
PHARMACOLGY HOMEWORK Overdose Management Add in important nursing notes for each of these medications and overdose management as well as administration route. Medication Overdose Nursing Notes (*Homework) Management (Routes) Acetaminophen Acetylcysteine Acetaminophen: (PO/PR) Route: PO, IV Max daily dose: 4000mg Acute toxicity (overdose) Monitor for S+S of hepatotoxicity (éLFTs, bilirubin, hypoglycemia, renal damage) Acetylcysteine: IV infusion: monitor for fluid overload and signs of hyponatremia such as changes in mental status Monitor for S+S of aspiration, bronchospasm, excess secretions Digitalis Digoxin Immune Fab Digoxin: (PO/IV) (Ovine, Digibind) Take apical pulse for 60sec. If < 60 BPM hold Route: IV digoxin dose and contact prescriber. Monitor serum digoxin (it has a narrow therapeutic index), potassium, magnesium, calcium. Monitor for S+S toxicity: anorexia, nausea, vomiting, diarrhea, visual disturbances, cardiac arrhythmias Digoxin Immune Fab: Skin allergy testing prior to administration for history of allergy or previous therapy of this drug Monitor cardiac status + rhythm, neurological status Toxicity reversal within an hour (adults), minutes (children) of antidote administration Monitor serum potassium, critical within first few hours; serum digoxin levels, ECG for 2-3 weeks post administration Heparin Protamine sulfate Heparin: (IV/SC) (IV) Monitor for spontaneous bleeding, thrombocytopenia Monitor aPTT levels Protamine Sulfate: Sudden drop in BP Monitor BP+ P q15-30 min Monitor aPTT Opioid Naloxone (IV-adults, Opioids: -

Calcium Gluconate
CALCIUM GLUCONATE CLASSIFICATION Minerals and electrolytes TRADE NAME(S) Calcium Gluconate 10% (for IV use) Calcium Gluconate gel 2.5% (for topical use) DESIRED EFFECTS Lower potassium levels; pain relief and neutralizing fluoride ion MECHANISM OF ACTION Calcium is the primary component of skeletal tissue. Bone serves as a calcium depot and as a reservoir for electrolytes and buffers. INDICATIONS Suspected hyperkalemia in adult PEA/Asystole Antidote for calcium channel blocker overdose and magnesium sulfate toxicity Gel is used for hydrofluoric acid burns Suspected hyperkalemia with adult crush injury or peaked T-waves on EKG CONTRAINDICATIONS Should not be given to patients with digitalis toxicity Should be used with caution in patients with dehydration ADVERSE REACTIONS When given too rapidly or to someone on digitalis, can cause sudden death from ventricular fibrillation May cause mild to severe IV site irritation SPECIAL CONSIDERATIONS Must either use a different IV line or flush line with copious normal saline if being given with sodium bicarbonate. When used on hydroflouric acid burns, relief of pain is the only indication of treatment efficacy. Therefore, use of analgesic agents is not recommended. DOSING REGIMEN Suspected hyperkalemia in adult PEA/asystole or adult crush injury, or evidence of EKG changes (Ex. peaked T-waves) o Calcium gluconate 10% 15-30 ml IV/IO over 2-5 minutes KNOWN calcium channel blocker overdose - administer 3 grams IV/IO may repeat dose in 10 minutes if no effect. Hydrofluoric acid burns apply calcium gluconate gel 2.5% every 15 minutes to burned area and massage continuously until pain disappears.