Volume-9, Issue-2, 2019/20 ISSN 2091-2854
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lockerbie Wildlife Trust Eskrigg Reserve August 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: SC 005538 August 2016 News Bulletin 1. View of pond on the 16th of August. 2. Confirmed wildlife sightings at the Reserve in August. a. Birds Blackbird, Black East Indian Duck, Blue Tit, Buzzard, Carrion Crow, Chaffinch, Coal Tit, Dunnock, Goldcrest, Goldfinch, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kingfisher, Little Grebe, Mallard, Moorhen, Nuthatch, Pheasant, Pied Flycatcher, Raven, Robin, Rook, Siskin, Song Thrush, Swallow, Treecreeper, Wood Pigeon, Wren. b. Mammals Bank Vole, Common Shrew, Fox, Hedgehog, Mole, Rabbit, Red Squirrel, Woodmouse. c. Reptiles and Amphibians Common Shrew - PC Hedgehog Common Lizard, Frog, Toad. d. Invertebrates Black Slug; Tree and White-tailed Bumble Bees; Common Carder Bee; Green-veined White, Large White, Meadow Brown, Painted Lady, Peacock, Red Admiral, Ringlet, Small Tortoiseshell and Small White butterflies; Crane-flies; Azure, Blue-tailed and Common Blue Damselflies; Common Darter, Common Hawker, Golden Ringed and Southern Hawker dragonflies; Froghoppers, Grasshoppers; Hoverflies; 7-Spot, 10-Spot and Larch Ladybirds; Midges; Mosquitoes; Common White Wave, Crescent, July Highflyer, LargeYellow Underwing and Silver-Y moths. 10-spot Ladybird Photographs: Jim Rae, Pam Copeland (PC). 1!! ! 3. August Photo-gallery. Row 1: Creeping Cinquefoil, Red Admiral Butterfly, Monkeyflower. Row 2: Peacock Butterfly, Fruiting Honeysuckle, Painted Lady Butterfly. Row 3: Dyer's Mazegill, Small Torytoiseshell Butterfly, Turkeytail. Row 4: Young Common Lizard, Common Carder Bee on Devil's-bit Scabious, Young Frog in reed-grass. Row 5: Burgundydrop Bonnet, Spider (Araneus diadematus), Yellow Stag's Horn. -

Plectological and Molecular Identification Of
Bangladesh J. Plant Taxon. 27(1): 67‒77, 2020 (June) © 2020 Bangladesh Association of Plant Taxonomists PLECTOLOGICAL AND MOLECULAR IDENTIFICATION OF ECONOMICALLY IMPORTANT WILD RUSSULALES MUSHROOMS FROM PAKISTAN AND THEIR ANTIFUNGAL POTENTIAL AGAINST FOOD PATHOGENIC FUNGUS ASPERGILLUS NIGER 1 SAMINA SARWAR*, TANZEELA AZIZ, MUHAMMAD HANIF , SOBIA ILYAS, 2 3 MALKA SABA , SANA KHALID AND MUHAMMAD FIAZ Department of Botany, Lahore College for Women University, Lahore, Pakistan Keywords: Aseptate; Biocontrol; Macrofungi; Micromycetes; Mycochemicals. Abstract Present study deals with the plectological and molecular analysis as well as use of economically important wild Russuloid mushrooms against food pathogenic fungus Aspergillus niger. Three different species of mushrooms viz., Russla laeta, R. nobilis, and R. nigricans were collected and identified from Himalayan range of Pakistan and are found as new records for this country. Major objective of this study was to highlight the importance of these wild creatures as antifungal agents against A. niger. For this purpose methanolic extract of selected mushrooms of different concentration levels viz., 1, 1.5, 2 and 3% were used. This activity is also first time reported from Pakistan by using this group of mushrooms. Results showed that all tested mushrooms exhibit growth inhibition of A. niger and can be used as biocontrol agents. R. nigricans showed maximum inhibition of fungus growth that is 62% at 3% concentrations while minimum inhibition was observed in R. nobilis at same concentration that is 43.6%. Introduction Many people in Pakistan depend on agriculture but various crops are contaminated by phytopathogenic fungi (i.e., Aspergillus, Fusarium, Penicillium) during pre and post-harvesting processes. -

Resumen Diversity and Distribution of Ganoderma
DAMIAN LÓPEZ-PEÑA1, ALDO GUTIÉRREZ1, EDUARDO HERNÁNDEZ-NAVARRO1, RICARDO VALENZUELA2 Y MARTÍN ESQUEDA1, 3 Resumen Se estudiaron siete especies de Ganoderma: G. applanatum, G. curtisii, G. lobatum, G. oerstedii, G. sessi- le, G. sessiliforme y G. weberianum. De ellas, G. lobatum y G. oerstedii son nuevos registros para Sonora, mientras que G. sessile y G. weberianum para México. El género tiene una amplia distribución en Sonora, en donde se encuentra en bosque de encino, encino-pino, pino-encino, encino abierto e incluso en zonas xeróflas con mezquital. Ganoderma oerstedii se encontró en la base de Stenocereus thurberi, que lo hace el primer registro sobre una cactácea. Palabras clave: corología, hongos poliporoides, sierra Sonorense, taxonomía. Diversity and distribution of Ganoderma (Polyporales: Ganodermataceae) from Sonora, Mexico Abstract Seven species of Ganoderma: G. applanatum, G. curtisii, G. lobatum, G oerstedii, G. sessile, G. sessilifor- me, and G. weberianum are discussed. Ganoderma lobatum and G. oerstedii are new records for Sonora, while G. sessile and G. weberianum for Mexico. The genus has a wide distribution in Sonora, being found in oak, oak-pine, pine-oak, open oak forests, also in xerophytic vegetation like mesquite scrub. Ganoder- ma oerstedii was founded on Stenocereus thurberi, being its frst record on a living Cactaceae. Key words: chorology, polyporoid fungi, Sonoran sierra, taxonomy. 1Centro de Investigación en Alimentación y Desarrollo, A.C. Hermosillo, Sonora, México. 2Escuela Nacional de Ciencias Biológicas, Instituto Politéc- nico Nacional. México, D.F., México. 3Autor para la corresponden- cia: [email protected] 431 DAMIAN LÓPEZ-PEÑA ET AL. anoderma P. Karst. (Polyporales: Ganodermataceae) es un género con más de 200 especies descritas, pero muchas son sinónimas (Moncalvo y Ryvarden, 1997; IFP, 2014). -

Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest
Asian Journal of Biology 9(2): 19-32, 2020; Article no.AJOB.55647 ISSN: 2456-7124 Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest D. R. B. Sonchita1, F. M. Aminuzzaman1*, A. A. Joty1, J. F. Tanni1, M. N. Islam1 and M. Rahaman1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration among all authors. Author DRBS collected the samples and conducted the research work. Author FMA designed and supervised the research work, collected the samples, wrote and edited the manuscript. Authors AAJ and MNI collected the samples. Authors JFT and MR managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJOB/2020/v9i230084 Editor(s): (1) Dr. P. Dhasarathan, Anna University, India. Reviewers: (1) Blagoy Uzunov, Sofia University “St. Kliment Ohridski”, Bulgaria. (2) Siddhant, Durgesh Nandini Degree College, India. (3) Shengrong Liu, Ningde Normal University, China. Complete Peer review History: http://www.sdiarticle4.com/review-history/55647 Received 20 March 2020 Original Research Article Accepted 27 May 2020 Published 05 June 2020 ABSTRACT Survey on macro fungi was made in Gajni forest, Sherpur, Bangladesh which is located in between 24°18' and 25°18' north latitudes and in between 89°53' and 90°91' east longitudes. It is bounded by Meghalaya state of India on the north, Mymensingh and Jamalpur districts on the south with a wide range of ecosystem. The survey was conducted on July to December, 2018 to identify and preserve wood-rot causal macro fungi for future industrial utilization. -

Phylogenetic Analysis of Widely Cultivated Ganoderma in China Based on the Mitochondrial V4-V6 Region of SSU Rdna
Phylogenetic analysis of widely cultivated Ganoderma in China based on the mitochondrial V4-V6 region of SSU rDNA X.W. Zhou1,2, K.Q. Su2 and Y.M. Zhang1 1School of Life and Environment Science, Shanghai Normal University, Shanghai, China 2Key Laboratory of Urban Agriculture (South) Ministry of Agriculture, Plant Biotechnology Research Center, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China Corresponding authors: Y.M. Zhang / X.W. Zhou E-mail: [email protected] / [email protected] Genet. Mol. Res. 14 (1): 886-897 (2015) Received February 6, 2014 Accepted May 15, 2014 Published February 2, 2015 DOI http://dx.doi.org/10.4238/2015.February.2.12 ABSTRACT. Ganoderma mushroom is one of the most prescribed traditional medicines and has been used for centuries, particularly in China, Japan, Korea, and other Asian countries. In this study, different strains of Ganoderma spp and the genetic relationships of the closely related strains were identified and investigated based on the V4-V6 region of mitochondrial small subunit ribosomal DNA of the Ganoderma species. The sizes of the mitochondrial ribosomal DNA regions from different Ganoderma species showed 2 types of sequences, 2.0 or 0.5 kb. A phylogenetic tree was constructed, which revealed a high level of genetic diversity in Ganoderma species. Ganoderma lucidum G05 and G. eupense G09 strains were clustered into a G. resinaceum group. Ganoderma spp G29 and G22 strains were clustered into a G. lucidum group. However, Ganoderma spp G19, G20, and G21 strains were Genetics and Molecular Research 14 (1): 886-897 (2015) ©FUNPEC-RP www.funpecrp.com.br Characterization of V4-V6 region of SSU rDNA of Ganoderma 887 clustered into a single group, the G. -

Diseases of Trees in the Great Plains
United States Department of Agriculture Diseases of Trees in the Great Plains Forest Rocky Mountain General Technical Service Research Station Report RMRS-GTR-335 November 2016 Bergdahl, Aaron D.; Hill, Alison, tech. coords. 2016. Diseases of trees in the Great Plains. Gen. Tech. Rep. RMRS-GTR-335. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 229 p. Abstract Hosts, distribution, symptoms and signs, disease cycle, and management strategies are described for 84 hardwood and 32 conifer diseases in 56 chapters. Color illustrations are provided to aid in accurate diagnosis. A glossary of technical terms and indexes to hosts and pathogens also are included. Keywords: Tree diseases, forest pathology, Great Plains, forest and tree health, windbreaks. Cover photos by: James A. Walla (top left), Laurie J. Stepanek (top right), David Leatherman (middle left), Aaron D. Bergdahl (middle right), James T. Blodgett (bottom left) and Laurie J. Stepanek (bottom right). To learn more about RMRS publications or search our online titles: www.fs.fed.us/rm/publications www.treesearch.fs.fed.us/ Background This technical report provides a guide to assist arborists, landowners, woody plant pest management specialists, foresters, and plant pathologists in the diagnosis and control of tree diseases encountered in the Great Plains. It contains 56 chapters on tree diseases prepared by 27 authors, and emphasizes disease situations as observed in the 10 states of the Great Plains: Colorado, Kansas, Montana, Nebraska, New Mexico, North Dakota, Oklahoma, South Dakota, Texas, and Wyoming. The need for an updated tree disease guide for the Great Plains has been recog- nized for some time and an account of the history of this publication is provided here. -

Department of Plant Pathology Dhaka-1207 June
OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH ARIFA AFRIN JOTY DEPARTMENT OF PLANT PATHOLOGY SHER-E-BANGLAAGRICULTURALUNIVERSITY DHAKA-1207 JUNE, 2018 OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH BY ARIFA AFRIN JOTY REGISTRATION NO. 12-05051 A Thesis Submitted to the Faculty of Agriculture Sher-e-Bangla Agricultural University, Dhaka-1207, in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE (M.S.) IN PLANT PATHOLOGY SEMESTER: JANUARY- JUNE, 2018 Approved by: Dr. F. M. Aminuzzaman Dr. Nazneen Sultana Professor Professor Department of Plant Pathology Department of Plant Pathology Sher-e-Bangla Agricultural University Sher-e-Bangla Agricultural University Supervisor Co- Supervisor Khadija Akhter Chairman Examination Committee ACKNOWLEDGEMENTS All admiration and praises are solely to “Almighty Allah” Whose mercy absolutely enabled the author to pursue the higher study in Agriculture discipline and complete M.S. Course and research work successfully for the degree of M.S. in Plant Pathology. The author expresses her immense respect and deepest sense of gratitude and heartfelt thanks to her most reverend teacher and Supervisor, Professor Dr. F. M. Aminuzzaman, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his untiring and efficient guidance, timely instruction, valuable advices and encouragement throughout the research work and completion of this thesis. The author is grateful to her research Co-Supervisor, Prof. Dr. Nazneen Sultana, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his guidance, keen interest, valuable advices and continuous encouragement regarding this research. The author also wishes to pay her deep respect to Prof. -
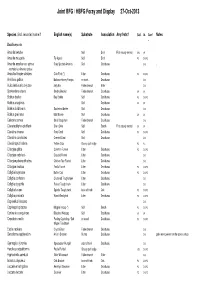
Joint BFG / HBFG Foray and Display 27-Oct-2013
Joint BFG / HBFG Foray and Display 27-Oct-2013 Species (incl. recorded name if English name(s) Substrate Association Any firsts? Coll. Id. Conf Notes . Basidiomycota Amanita betulae Soil Birch First county record SH3 SK Amanita muscaria Fly Agaric Soil Birch PC DJSPC Amanita excelsa var. spissa Grey Spotted Amanita Soil Deciduous DJS . recorded as Amanita spissa Ampulloclitocybe clavipes Club Foot (*) Litter Deciduous PC DJSPC . Armillaria gallica Bulbous Honey Fungus on roots Deciduous DJS Auricularia auricula-judae Jelly Ear Fallen branch Elder DJS Bjerkandera adusta Smoky Bracket Fallen branch Deciduous SK SK Boletus badius Bay Bolete Soil Deciduous PC DJSPC Boletus cisalpinus Soil Deciduous SK SK Boletus luridiformis Scarletina Bolete Soil Deciduous DJS Boletus pruinatus Matt Bolete Soil Deciduous SK SK Calocera cornea Small Stagshorn Fallen branch Deciduous DJS Clavariadelphus pistillaris Giant Club Soil Beech First county record SK SK Clavulina cinerea Grey Coral Soil Deciduous PC DJSPC Clavulina coralloides Crested Coral Soil Deciduous DJS Clavulinopsis helvola Yellow Club Grassy path edge PC PC Clitocybe gibba Common Funnel Litter Deciduous PC DJSPC Clitocybe nebularis Clouded Funnel Litter Deciduous DJS Clitocybe phaeophthalma Chicken Run Funnel Litter Deciduous DJS Clitocybe rivulosa Fool's Funnel Litter Deciduous PC DJSPC Collybia butyracea Butter Cap Litter Deciduous PC DJSPC Collybia confluens Clustered Toughshank Litter Deciduous DJS Collybia dryophila Russet Toughshank Litter Deciduous DJS Collybia fusipes Spindle Toughshank -

Polypore Diversity in North America with an Annotated Checklist
Mycol Progress (2016) 15:771–790 DOI 10.1007/s11557-016-1207-7 ORIGINAL ARTICLE Polypore diversity in North America with an annotated checklist Li-Wei Zhou1 & Karen K. Nakasone2 & Harold H. Burdsall Jr.2 & James Ginns3 & Josef Vlasák4 & Otto Miettinen5 & Viacheslav Spirin5 & Tuomo Niemelä 5 & Hai-Sheng Yuan1 & Shuang-Hui He6 & Bao-Kai Cui6 & Jia-Hui Xing6 & Yu-Cheng Dai6 Received: 20 May 2016 /Accepted: 9 June 2016 /Published online: 30 June 2016 # German Mycological Society and Springer-Verlag Berlin Heidelberg 2016 Abstract Profound changes to the taxonomy and classifica- 11 orders, while six other species from three genera have tion of polypores have occurred since the advent of molecular uncertain taxonomic position at the order level. Three orders, phylogenetics in the 1990s. The last major monograph of viz. Polyporales, Hymenochaetales and Russulales, accom- North American polypores was published by Gilbertson and modate most of polypore species (93.7 %) and genera Ryvarden in 1986–1987. In the intervening 30 years, new (88.8 %). We hope that this updated checklist will inspire species, new combinations, and new records of polypores future studies in the polypore mycota of North America and were reported from North America. As a result, an updated contribute to the diversity and systematics of polypores checklist of North American polypores is needed to reflect the worldwide. polypore diversity in there. We recognize 492 species of polypores from 146 genera in North America. Of these, 232 Keywords Basidiomycota . Phylogeny . Taxonomy . species are unchanged from Gilbertson and Ryvarden’smono- Wood-decaying fungus graph, and 175 species required name or authority changes. -

Tarset and Greystead Biological Records
Tarset and Greystead Biological Records published by the Tarset Archive Group 2015 Foreword Tarset Archive Group is delighted to be able to present this consolidation of biological records held, for easy reference by anyone interested in our part of Northumberland. It is a parallel publication to the Archaeological and Historical Sites Atlas we first published in 2006, and the more recent Gazeteer which both augments the Atlas and catalogues each site in greater detail. Both sets of data are also being mapped onto GIS. We would like to thank everyone who has helped with and supported this project - in particular Neville Geddes, Planning and Environment manager, North England Forestry Commission, for his invaluable advice and generous guidance with the GIS mapping, as well as for giving us information about the archaeological sites in the forested areas for our Atlas revisions; Northumberland National Park and Tarset 2050 CIC for their all-important funding support, and of course Bill Burlton, who after years of sharing his expertise on our wildflower and tree projects and validating our work, agreed to take this commission and pull everything together, obtaining the use of ERIC’s data from which to select the records relevant to Tarset and Greystead. Even as we write we are aware that new records are being collected and sites confirmed, and that it is in the nature of these publications that they are out of date by the time you read them. But there is also value in taking snapshots of what is known at a particular point in time, without which we have no way of measuring change or recognising the hugely rich biodiversity of where we are fortunate enough to live. -

Biosorption of Cu(II) from Aqueous Solutions by a Macrofungus (Ganoderma Lobatum) Biomass and Its Biochar
p-ISSN: 0972-6268 Nature Environment and Pollution Technology (Print copies up to 2016) Vol. 20 No. 2 pp. 579-585 2021 An International Quarterly Scientific Journal e-ISSN: 2395-3454 Original Research Paper Originalhttps://doi.org/10.46488/NEPT.2021.v20i02.014 Research Paper Open Access Journal Biosorption of Cu(II) from Aqueous Solutions by a Macrofungus (Ganoderma lobatum) Biomass and its Biochar Silin Yang, Yan Wang and Yungen Liu† Faculty of Ecology and Environment, Southwest Forestry University, Kunming 650224, China †Corresponding author: Yungen Liu; [email protected] ABSTRACT Nat. Env. & Poll. Tech. Website: www.neptjournal.com The sorption capacities of the macrofungus viz. Ganoderma lobatum (C0) and its biochar (C400) were evaluated for the biosorption of Cu(II) from aqueous solution under different conditions, including Received: 18-03-2020 adsorbent doses, pH of the solution, contact time and initial Cu(II) concentration. The results showed Revised: 25-04-2020 Accepted: 15-06-2020 that Ganoderma lobatum could be used as an efficient biosorbent for the removal of Cu(II) ions from an aqueous solution. The desired biosorbent dose in the case of C0 and C400 for Cu(II) adsorption Key Words: was 4 g/L, and the optimal pH value for biosorption was 8 for Cu(II). The Freundlich isotherm model Macrofungus fitted the absorption data of Cu(II) for both C0 and C400 better than the Langmuir isotherm model, and Biochar the adsorption capacity of C0 was better than C400. Our results indicate that C0 has a higher removal Sorption efficiency than C400 in adsorbing Cu(II) ions from aqueous solution. -

Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka
Journal of Advances in Biology & Biotechnology 11(4): 1-18, 2017; Article no.JABB.30970 ISSN: 2394-1081 SCIENCEDOMAIN international www.sciencedomain.org Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka H. Rubina1, F. M. Aminuzzaman1*, M. S. M. Chowdhury1 and K. Das1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration between all authors. Author HR wrote the protocol, carried out research and wrote the first draft of the manuscript. Author KD carried out microscopic work and spore morphology. Author FMA designed and supervised the research, identified the fungal genera and also edited the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/JABB/2017/30970 Editor(s): (1) Mohammad Arif, Department of Plant Pathology, Kansas State University, Manhattan, Kansas, USA. Reviewers: (1) Chitta Ranjan Deb, Nagaland University, Lumami, India. (2) Rajesh Kumar, Rain Forest Research Institute, Assam, India. Complete Peer review History: http://www.sciencedomain.org/review-history/17933 Received 12th December 2016 th Original Research Article Accepted 6 February 2017 Published 23rd February 2017 ABSTRACT This investigation was conducted in National Botanical Garden, Dhaka located at 24°00′ N (Latitude), 90°00′ E (Longitude) to document the morphology, diversity and distribution of macro fungi during the rainy seasons of July to October, 2015. A total of 23 macro fungi samples were collected and identified to 20 species under 10 genera and 10 families. The predominant genera were Ganoderma sp., Lepiota sp., Daedeleopsis sp., Russula sp., Psythyrella sp., Lycoperdon sp., Crepidotus sp., Psilocybe sp, Flammulina sp.