Plectological and Molecular Identification Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lockerbie Wildlife Trust Eskrigg Reserve August 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: SC 005538 August 2016 News Bulletin 1. View of pond on the 16th of August. 2. Confirmed wildlife sightings at the Reserve in August. a. Birds Blackbird, Black East Indian Duck, Blue Tit, Buzzard, Carrion Crow, Chaffinch, Coal Tit, Dunnock, Goldcrest, Goldfinch, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kingfisher, Little Grebe, Mallard, Moorhen, Nuthatch, Pheasant, Pied Flycatcher, Raven, Robin, Rook, Siskin, Song Thrush, Swallow, Treecreeper, Wood Pigeon, Wren. b. Mammals Bank Vole, Common Shrew, Fox, Hedgehog, Mole, Rabbit, Red Squirrel, Woodmouse. c. Reptiles and Amphibians Common Shrew - PC Hedgehog Common Lizard, Frog, Toad. d. Invertebrates Black Slug; Tree and White-tailed Bumble Bees; Common Carder Bee; Green-veined White, Large White, Meadow Brown, Painted Lady, Peacock, Red Admiral, Ringlet, Small Tortoiseshell and Small White butterflies; Crane-flies; Azure, Blue-tailed and Common Blue Damselflies; Common Darter, Common Hawker, Golden Ringed and Southern Hawker dragonflies; Froghoppers, Grasshoppers; Hoverflies; 7-Spot, 10-Spot and Larch Ladybirds; Midges; Mosquitoes; Common White Wave, Crescent, July Highflyer, LargeYellow Underwing and Silver-Y moths. 10-spot Ladybird Photographs: Jim Rae, Pam Copeland (PC). 1!! ! 3. August Photo-gallery. Row 1: Creeping Cinquefoil, Red Admiral Butterfly, Monkeyflower. Row 2: Peacock Butterfly, Fruiting Honeysuckle, Painted Lady Butterfly. Row 3: Dyer's Mazegill, Small Torytoiseshell Butterfly, Turkeytail. Row 4: Young Common Lizard, Common Carder Bee on Devil's-bit Scabious, Young Frog in reed-grass. Row 5: Burgundydrop Bonnet, Spider (Araneus diadematus), Yellow Stag's Horn. -

Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest
Asian Journal of Biology 9(2): 19-32, 2020; Article no.AJOB.55647 ISSN: 2456-7124 Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest D. R. B. Sonchita1, F. M. Aminuzzaman1*, A. A. Joty1, J. F. Tanni1, M. N. Islam1 and M. Rahaman1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration among all authors. Author DRBS collected the samples and conducted the research work. Author FMA designed and supervised the research work, collected the samples, wrote and edited the manuscript. Authors AAJ and MNI collected the samples. Authors JFT and MR managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJOB/2020/v9i230084 Editor(s): (1) Dr. P. Dhasarathan, Anna University, India. Reviewers: (1) Blagoy Uzunov, Sofia University “St. Kliment Ohridski”, Bulgaria. (2) Siddhant, Durgesh Nandini Degree College, India. (3) Shengrong Liu, Ningde Normal University, China. Complete Peer review History: http://www.sdiarticle4.com/review-history/55647 Received 20 March 2020 Original Research Article Accepted 27 May 2020 Published 05 June 2020 ABSTRACT Survey on macro fungi was made in Gajni forest, Sherpur, Bangladesh which is located in between 24°18' and 25°18' north latitudes and in between 89°53' and 90°91' east longitudes. It is bounded by Meghalaya state of India on the north, Mymensingh and Jamalpur districts on the south with a wide range of ecosystem. The survey was conducted on July to December, 2018 to identify and preserve wood-rot causal macro fungi for future industrial utilization. -

Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity
molecules Article Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity Marina Kosti´c 1 , Marija Ivanov 1 , Ângela Fernandes 2 , José Pinela 2 , Ricardo C. Calhelha 2 , Jasmina Glamoˇclija 1, Lillian Barros 2 , Isabel C. F. R. Ferreira 2 , Marina Sokovi´c 1,* and Ana Ciri´c´ 1,* 1 Department of Plant Physiology, Institute for Biological Research “Siniša Stankovi´c”-National Institute of Republic of Serbia, University of Belgrade, Bulevar Despota Stefana 142, 11000 Belgrade, Serbia; [email protected] (M.K.); [email protected] (M.I.); [email protected] (J.G.) 2 Centro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal; [email protected] (Â.F.); [email protected] (J.P.); [email protected] (R.C.C.); [email protected] (L.B.); [email protected] (I.C.F.R.F.) * Correspondence: [email protected] (M.S.); [email protected] (A.C.);´ Fax: +381-11-207-84-33 (M.S. & A.C.)´ Academic Editor: Laura De Martino Received: 6 September 2020; Accepted: 20 September 2020; Published: 22 September 2020 Abstract: This study explored the biological properties of three wild growing Russula species (R. integra, R. rosea, R. nigricans) from Serbia. Compositional features and antioxidant, antibacterial, antibiofilm, and cytotoxic activities were analyzed. The studied mushroom species were identified as being rich sources of carbohydrates and of low caloric value. Mannitol was the most abundant free sugar and quinic and malic acids the major organic acids detected. The four tocopherol isoforms were found, and polyunsaturated fatty acids were the predominant fat constituents. -

Sayı Tam Dosyası
']FHhQLYHUVLWHVL2UPDQFÕOÕN'HUJLVL&LOW166D\Õ2 )DNOWH$GÕQD6DKLEL : 3URI'U+DOGXQ0h'(55ø62ö/8 %Dú(GLW|U : 'Ro'U(QJLQ(52ö/8 Editör Kurulu Alan Editörleri Prof. Dr. Oktay YILDIZ 3URI'U'HU\D(ù(1 Prof. Dr. Kermit CROMAC Jr. (Oregon State University) Prof. Dr. Rimvydas VASAITIS (Swedish University of Agricultural Sciences) 3URI'U-LĜt5(0(â &]HFK8QLYHUVLW\RI/LIH6FLHQFHV3UDJXH Prof. Dr. Marc J. LINIT (University of Missouri) 3URI'U=HNL'(0ø5 Prof. Dr. (PUDKdød(. Prof. 'U'U'HU\D6(9ø0.25.87 Prof. 'U$\ELNH$\IHU.$5$'$ö Doç'U0.ÕYDQo$. Doç'U7DUÕN*('ø. Doç. Dr. Akif KETEN Doç. Dr. Ali Kemal ÖZBAYRAM 'UgJUh3ÕQDU.g</h 'UgJUh'U+DVDQg='(0ø5 Dr. Ögr. Ü. Dr. Hüseyin AMBARLI Dr. gJUh'UøGULV'85862< 'UgJUh'U%LODOd(7ø1 Teknik Editörler $Uú*|U6HUWDo.$<$ $Uú*|U0XKDPPHWdø/ $Uú*|U'UdD÷ODU$.d$< $Uú*|U'U7DUÕNdø7*(= Dr. Ögr. Ü. Ömer ÖZYÜREK $Uú*|U1XUD\g=7h5. $Uú*|U<ÕOGÕ]%$+d(&ø $Uú*|UAbdullah Hüseyin DÖNMEZ Dil Editörleri gJU*|U'UøVPDLO.2d Ögr. Gör. Dr. Zennure UÇAR zĂnjŦƔŵĂĚƌĞƐŝ ŽƌƌĞƐƉŽŶĚŝŶŐĚĚƌĞƐƐ Düzce Üniversitesi Duzce University Orman Fakültesi Faculty of Forestry ϴϭϲϮϬ<ŽŶƵƌĂůƉzĞƌůĞƔŬĞƐŝͬƺnjĐĞ-dmZ<7z ϴϭϲϮϬ<ŽŶƵƌĂůƉĂŵƉƵƐͬƺnjĐĞ-dhZ<z 'HUJL\ÕOGDLNLVD\ÕRODUDN\D\ÕQODQÕU 7KLVMRXUQDOLVSXEOLVKHGVHPLDQQXDOO\ http://www.duzce.edu.tr/of/ DGUHVLQGHQGHUJL\HLOLúNLQELOJLOHUHYHPDNDOH|]HWOHULQHXODúÕODELOLU (Instructions to Authors" and "Abstracts" can be found at this address). ødø1'(.ø/(5 +X]XUHYL%DKoHOHULQLQ<Dú'RVWX7DVDUÕP$oÕVÕQGDQøQFHOHQPHVLAntalya-7UNL\HgUQH÷L«««««1 Tahsin YILMAZ, Bensu YÜCE .HQWVHO5HNUHDV\RQHO$ODQODUGDNL%LWNL9DUOÕ÷Õ5L]HgUQH÷L«««««««««««««««««16 Ömer Lütfü ÇORBACI, *|NKDQ$%$<7UNHU2ö8=7h5.0HUYHhd2. <Õ÷ÕOFD ']FH %DON|\ %DO2UPDQÕ)ORUDVÕ««««««««««««««««««««««««45 (OLI$\úH<,/',5,01HYDO*h1(ùg=.$11XUJO.$5/,2ö/8.,/,d Assessment of Basic Green Infrastructure Components as Part of Landscape Structure for Siirt……...70 Huriye Simten SÜTÜNÇ, Ömer Lütfü ÇORBACI Cephalaria duzceënsis N. -

Forest Fungi in Ireland
FOREST FUNGI IN IRELAND PAUL DOWDING and LOUIS SMITH COFORD, National Council for Forest Research and Development Arena House Arena Road Sandyford Dublin 18 Ireland Tel: + 353 1 2130725 Fax: + 353 1 2130611 © COFORD 2008 First published in 2008 by COFORD, National Council for Forest Research and Development, Dublin, Ireland. All rights reserved. No part of this publication may be reproduced, or stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying recording or otherwise, without prior permission in writing from COFORD. All photographs and illustrations are the copyright of the authors unless otherwise indicated. ISBN 1 902696 62 X Title: Forest fungi in Ireland. Authors: Paul Dowding and Louis Smith Citation: Dowding, P. and Smith, L. 2008. Forest fungi in Ireland. COFORD, Dublin. The views and opinions expressed in this publication belong to the authors alone and do not necessarily reflect those of COFORD. i CONTENTS Foreword..................................................................................................................v Réamhfhocal...........................................................................................................vi Preface ....................................................................................................................vii Réamhrá................................................................................................................viii Acknowledgements...............................................................................................ix -

Department of Plant Pathology Dhaka-1207 June
OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH ARIFA AFRIN JOTY DEPARTMENT OF PLANT PATHOLOGY SHER-E-BANGLAAGRICULTURALUNIVERSITY DHAKA-1207 JUNE, 2018 OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH BY ARIFA AFRIN JOTY REGISTRATION NO. 12-05051 A Thesis Submitted to the Faculty of Agriculture Sher-e-Bangla Agricultural University, Dhaka-1207, in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE (M.S.) IN PLANT PATHOLOGY SEMESTER: JANUARY- JUNE, 2018 Approved by: Dr. F. M. Aminuzzaman Dr. Nazneen Sultana Professor Professor Department of Plant Pathology Department of Plant Pathology Sher-e-Bangla Agricultural University Sher-e-Bangla Agricultural University Supervisor Co- Supervisor Khadija Akhter Chairman Examination Committee ACKNOWLEDGEMENTS All admiration and praises are solely to “Almighty Allah” Whose mercy absolutely enabled the author to pursue the higher study in Agriculture discipline and complete M.S. Course and research work successfully for the degree of M.S. in Plant Pathology. The author expresses her immense respect and deepest sense of gratitude and heartfelt thanks to her most reverend teacher and Supervisor, Professor Dr. F. M. Aminuzzaman, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his untiring and efficient guidance, timely instruction, valuable advices and encouragement throughout the research work and completion of this thesis. The author is grateful to her research Co-Supervisor, Prof. Dr. Nazneen Sultana, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his guidance, keen interest, valuable advices and continuous encouragement regarding this research. The author also wishes to pay her deep respect to Prof. -

<I>Russula Atroaeruginea</I> and <I>R. Sichuanensis</I> Spp. Nov. from Southwest China
ISSN (print) 0093-4666 © 2013. Mycotaxon, Ltd. ISSN (online) 2154-8889 MYCOTAXON http://dx.doi.org/10.5248/124.173 Volume 124, pp. 173–188 April–June 2013 Russula atroaeruginea and R. sichuanensis spp. nov. from southwest China Guo-Jie Li1,2, Qi Zhao3, Dong Zhao1, Shuang-Fen Yue1,4, Sai-Fei Li1, Hua-An Wen1a* & Xing-Zhong Liu1b* 1State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, No. 1 Beichen West Road, Chaoyang District, Beijing 100101, China 2University of Chinese Academy of Sciences, Beijing 100049, China 3Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, Kunming 650204, Yunnan, China 4College of Life Science, Capital Normal University, Xisihuanbeilu 105, Haidian District, Beijing 100048, China * Correspondence to: a [email protected] b [email protected] Abstract — Two new species of Russula are described from southwestern China based on morphology and ITS1-5.8S-ITS2 rDNA sequence analysis. Russula atroaeruginea (sect. Griseinae) is characterized by a glabrous dark-green and radially yellowish tinged pileus, slightly yellowish context, spores ornamented by low warts linked by fine lines, and numerous pileocystidia with crystalline contents blackening in sulfovanillin. Russula sichuanensis, a semi-sequestrate taxon closely related to sect. Laricinae, forms russuloid to secotioid basidiocarps with yellowish to orange sublamellate gleba and large basidiospores with warts linked as ridges. The rDNA ITS-based phylogenetic trees fully support these new species. Key words — taxonomy, Macowanites, Russulales, Russulaceae, Basidiomycota Introduction Russula Pers. is a globally distributed genus of macrofungi with colorful fruit bodies (Bills et al. 1986, Singer 1986, Miller & Buyck 2002, Kirk et al. -

Toxic Fungi of Western North America
Toxic Fungi of Western North America by Thomas J. Duffy, MD Published by MykoWeb (www.mykoweb.com) March, 2008 (Web) August, 2008 (PDF) 2 Toxic Fungi of Western North America Copyright © 2008 by Thomas J. Duffy & Michael G. Wood Toxic Fungi of Western North America 3 Contents Introductory Material ........................................................................................... 7 Dedication ............................................................................................................... 7 Preface .................................................................................................................... 7 Acknowledgements ................................................................................................. 7 An Introduction to Mushrooms & Mushroom Poisoning .............................. 9 Introduction and collection of specimens .............................................................. 9 General overview of mushroom poisonings ......................................................... 10 Ecology and general anatomy of fungi ................................................................ 11 Description and habitat of Amanita phalloides and Amanita ocreata .............. 14 History of Amanita ocreata and Amanita phalloides in the West ..................... 18 The classical history of Amanita phalloides and related species ....................... 20 Mushroom poisoning case registry ...................................................................... 21 “Look-Alike” mushrooms ..................................................................................... -

Mycelial Cultivation of 4 Edible Mushrooms from Khao Kra-Dong Volcano Forest Park, Thailand Tepupsorn Saensuk1* and Suteera Suntararak2
Naresuan University Journal: Science and Technology 2018; (26)2 Mycelial Cultivation of 4 Edible Mushrooms from Khao Kra-Dong Volcano Forest Park, Thailand Tepupsorn Saensuk1* and Suteera Suntararak2 1Lecturer, Department of Biology, Faculty of Science, Buriram Rajabhat University, 31000 Thailand 2Assistant Professor, Department of Environmental Science, Faculty of Science, Buriram Rajabhat University, 31000 Thailand * Corresponding Author, E-mail address: [email protected] Received: 4 May 2017; Accepted: 10 August 2017 Abstract The edible mushrooms become one of the world’s most expensive foods and have a global market measured in Thailand. In Thailand, the fruiting body of all occurs once a year during rainy season in June – August. So, the objective of this research was to study the optimal mycelial conditions of 4 edible mushrooms collected from Khao Kra-Dong Volcano Forest Park in Thailand: Russula cyanoxantha, Heimiell retispora, Russula virescens and Boletus colossus. The highest mycelial growth of R. virescens and B. colossus were with potato dextrose agar (PDA), followed by potato dextrose agar with 2% volcanic soil (PDA+2% S). The best structures of R. cyanoxantha and H. retispora used for culturing on medium were cap, stalk and spore, respectively. For R. Virescens and B. colossus, the best structures used for culturing on medium were stalk, cap and spore, respectively. The highest colony diameter of R. Cyanoxantha on PDA+2% S with cap was 68.00+1.00 mm. For H. retispora, the highest colony diameters on PDA+2%S with cap and PDA with stalk were 87.00+1.00 mm. and 67.33+1.53 mm., respectively. -
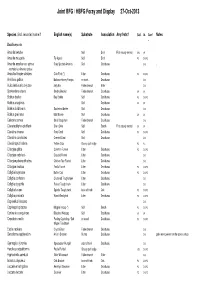
Joint BFG / HBFG Foray and Display 27-Oct-2013
Joint BFG / HBFG Foray and Display 27-Oct-2013 Species (incl. recorded name if English name(s) Substrate Association Any firsts? Coll. Id. Conf Notes . Basidiomycota Amanita betulae Soil Birch First county record SH3 SK Amanita muscaria Fly Agaric Soil Birch PC DJSPC Amanita excelsa var. spissa Grey Spotted Amanita Soil Deciduous DJS . recorded as Amanita spissa Ampulloclitocybe clavipes Club Foot (*) Litter Deciduous PC DJSPC . Armillaria gallica Bulbous Honey Fungus on roots Deciduous DJS Auricularia auricula-judae Jelly Ear Fallen branch Elder DJS Bjerkandera adusta Smoky Bracket Fallen branch Deciduous SK SK Boletus badius Bay Bolete Soil Deciduous PC DJSPC Boletus cisalpinus Soil Deciduous SK SK Boletus luridiformis Scarletina Bolete Soil Deciduous DJS Boletus pruinatus Matt Bolete Soil Deciduous SK SK Calocera cornea Small Stagshorn Fallen branch Deciduous DJS Clavariadelphus pistillaris Giant Club Soil Beech First county record SK SK Clavulina cinerea Grey Coral Soil Deciduous PC DJSPC Clavulina coralloides Crested Coral Soil Deciduous DJS Clavulinopsis helvola Yellow Club Grassy path edge PC PC Clitocybe gibba Common Funnel Litter Deciduous PC DJSPC Clitocybe nebularis Clouded Funnel Litter Deciduous DJS Clitocybe phaeophthalma Chicken Run Funnel Litter Deciduous DJS Clitocybe rivulosa Fool's Funnel Litter Deciduous PC DJSPC Collybia butyracea Butter Cap Litter Deciduous PC DJSPC Collybia confluens Clustered Toughshank Litter Deciduous DJS Collybia dryophila Russet Toughshank Litter Deciduous DJS Collybia fusipes Spindle Toughshank -

Download Full Article in PDF Format
Cryptogamie, Mycologie, 2017, 38 (2): 191-203 © 2017 Adac. Tous droits réservés Russula dinghuensis sp. nov. and R. subpallidirosea sp. nov., two new species from southern China supported by morphological and molecular evidence Jianbin ZHANG, Jingwei LI, Fang Li &Lihong QIU* State Key Lab of Biocontrol, School of Life Science, Sun Yat-sen University,Guangzhou 510275, China Abstract –Two new taxa of Russula from the Dinghu Mountain, Guangdong Province, China were described and illustrated based on both morphological data and phylogenetic analysis of the internal transcribed spacer sequences. Russula dinghuensis is characterized by the olive green pileus, acute and incurved margin, white and rarely forked lamellae, white spore print, globose to ellipsoid basidiospores with stocky and isolated warts, thick metachromatic pileipellis, and slender,furcated and septated terminal elements of pileipellis. Russula subpallidirosea is recognized by the pale pink to pale grayish-pink pileus, white and forked lamellae, white spore print, subglobose to ellipsoid basidiospores with the isolated, subcylindrical to conical warts, the metachromatic pileipellis, and the short, furcated and septate terminal elements of pileipellis. Both molecular and morphological analyses consistently confirm that these two new taxa are placed into Russula subg. Heterophyllidia subsection Cyanoxanthinae. The morphological differences among these two novel species and the closely related taxa are discussed. Cyanoxanthinae /Dinghu Mountain /ITS /phylogeny /taxonomy INTRODUCTION -

Journal of Botany
ACADEMY OF SCIENCES OF MOLDOVA BOTANICAL GARDEN (INSTITUTE) JOURNAL OF BOTANY VOL. VII NR. 2(11) Chisinau, 2015 FOUNDER OF THE "JOURNAL OF BOTANY": BOTANICAL GARDEN (INSTITUTE) OF THE ACADEMY OF SCIENCES OF MOLDOVA According to the decision of Supreme Council for Sciences and Technological Development of ASM and National Council for Accreditation and Attestation, nr. 288 of 28.11.2013 on the approval of the assessment and Classification of scientific journals, "Journal of Botany" was granted the status of scientific publication of "B" Category. EDITORIAL BOARD OF THE "JOURNAL OF BOTANY" Ciubotaru Alexandru, academician of the ASM, editor-in-chef, Botanical Garden (Institute) of the ASM Teleuță Alexandru, Ph.D., associete editor, Botanical Garden (Institute) of the ASM Cutcovschi-Muștuc Alina, Ph.D., secretary, Botanical Garden (Institute) of the ASM Members: Duca Gheorghe, academician of the ASM, President of the Academy of Sciences of Moldova Dediu Ion, corresponding member of the ASM, Institute of Geography and Ecology of the ASM Șalaru Vasile, corresponding member of the ASM, Moldova State University Colțun Maricica, Ph. D., Botanical Garden (Institute) of the ASM Tănase Cătălin, university professor, Botanical Garden "A. Fătu" of the "Al. I. Cuza" University, Iași, România Cristea Vasile, university professor, Botanical Garden "Alexandru Borza" of the "Babeș-Bolyai" University, Cluj-Napoca, România Toma Constantin, university professor, "Al. I. Cuza" University, Iași, România Sârbu Anca, university professor, Botanical Garden "D.Brîndza", Bucharest, România Zaimenco Natalia, university professor, M.M.Grishko National Botanical Garden of National Academy of Sciences of Ukraine Edition supported by the Supreme Council for Sciences and Technological Development of ASM Botanical Garden (Institute) MD - 2002, Padurii str.