Rog Et Al 2020 Mycorrhizal Carbon Sharing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lockerbie Wildlife Trust Eskrigg Reserve August 2016 News Bulletin
Lockerbie Wildlife Trust (www.lockerbie-wildlife-trust.co.uk) Eskrigg Reserve Scottish Charity No: SC 005538 August 2016 News Bulletin 1. View of pond on the 16th of August. 2. Confirmed wildlife sightings at the Reserve in August. a. Birds Blackbird, Black East Indian Duck, Blue Tit, Buzzard, Carrion Crow, Chaffinch, Coal Tit, Dunnock, Goldcrest, Goldfinch, Great Spotted Woodpecker, Great Tit, Greenfinch, Grey Heron, Grey Wagtail, House Martin, House Sparrow, Jackdaw, Jay, Kingfisher, Little Grebe, Mallard, Moorhen, Nuthatch, Pheasant, Pied Flycatcher, Raven, Robin, Rook, Siskin, Song Thrush, Swallow, Treecreeper, Wood Pigeon, Wren. b. Mammals Bank Vole, Common Shrew, Fox, Hedgehog, Mole, Rabbit, Red Squirrel, Woodmouse. c. Reptiles and Amphibians Common Shrew - PC Hedgehog Common Lizard, Frog, Toad. d. Invertebrates Black Slug; Tree and White-tailed Bumble Bees; Common Carder Bee; Green-veined White, Large White, Meadow Brown, Painted Lady, Peacock, Red Admiral, Ringlet, Small Tortoiseshell and Small White butterflies; Crane-flies; Azure, Blue-tailed and Common Blue Damselflies; Common Darter, Common Hawker, Golden Ringed and Southern Hawker dragonflies; Froghoppers, Grasshoppers; Hoverflies; 7-Spot, 10-Spot and Larch Ladybirds; Midges; Mosquitoes; Common White Wave, Crescent, July Highflyer, LargeYellow Underwing and Silver-Y moths. 10-spot Ladybird Photographs: Jim Rae, Pam Copeland (PC). 1!! ! 3. August Photo-gallery. Row 1: Creeping Cinquefoil, Red Admiral Butterfly, Monkeyflower. Row 2: Peacock Butterfly, Fruiting Honeysuckle, Painted Lady Butterfly. Row 3: Dyer's Mazegill, Small Torytoiseshell Butterfly, Turkeytail. Row 4: Young Common Lizard, Common Carder Bee on Devil's-bit Scabious, Young Frog in reed-grass. Row 5: Burgundydrop Bonnet, Spider (Araneus diadematus), Yellow Stag's Horn. -

Diversity and Phylogeny of Suillus (Suillaceae; Boletales; Basidiomycota) from Coniferous Forests of Pakistan
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY ISSN Print: 1560–8530; ISSN Online: 1814–9596 13–870/2014/16–3–489–497 http://www.fspublishers.org Full Length Article Diversity and Phylogeny of Suillus (Suillaceae; Boletales; Basidiomycota) from Coniferous Forests of Pakistan Samina Sarwar * and Abdul Nasir Khalid Department of Botany, University of the Punjab, Quaid-e-Azam Campus, Lahore, 54950, Pakistan *For correspondence: [email protected] Abstract Suillus (Boletales; Basidiomycota) is an ectomycorrhizal genus, generally associated with Pinaceae. Coniferous forests of Pakistan are rich in mycodiversity and Suillus species are found as early appearing fungi in the vicinity of conifers. This study reports the diversity of Suillus collected during a period of three (3) years (2008-2011). From 32 basidiomata of Suillus collected, 12 species of this genus were identified. These basidiomata were characterized morphologically, and phylogenetically by amplifying and sequencing the ITS region of rDNA. © 2014 Friends Science Publishers Keywords: Moist temperate forests; PCR; rDNA; Ectomycorrhizae Introduction adequate temperature make the environment suitable for the growth of mushrooms in these forests. Suillus (Suillaceae, Basidiomycota, Boletales ) forms This paper described the diversity of Suillus (Boletes, ectomycorrhizal associations mostly with members of the Fungi) with the help of the anatomical, morphological and Pinaceae and is characterized by having slimy caps, genetic analyses as little knowledge is available from forests glandular dots on the stipe, large pore openings that are in Pakistan. often arranged radially and a partial veil that leaves a ring or tissue hanging from the cap margin (Kuo, 2004). This genus Materials and Methods is mostly distributed in northern temperate locations, although some species have been reported in the southern Sporocarp Collection hemisphere as well (Kirk et al ., 2008). -

Plectological and Molecular Identification Of
Bangladesh J. Plant Taxon. 27(1): 67‒77, 2020 (June) © 2020 Bangladesh Association of Plant Taxonomists PLECTOLOGICAL AND MOLECULAR IDENTIFICATION OF ECONOMICALLY IMPORTANT WILD RUSSULALES MUSHROOMS FROM PAKISTAN AND THEIR ANTIFUNGAL POTENTIAL AGAINST FOOD PATHOGENIC FUNGUS ASPERGILLUS NIGER 1 SAMINA SARWAR*, TANZEELA AZIZ, MUHAMMAD HANIF , SOBIA ILYAS, 2 3 MALKA SABA , SANA KHALID AND MUHAMMAD FIAZ Department of Botany, Lahore College for Women University, Lahore, Pakistan Keywords: Aseptate; Biocontrol; Macrofungi; Micromycetes; Mycochemicals. Abstract Present study deals with the plectological and molecular analysis as well as use of economically important wild Russuloid mushrooms against food pathogenic fungus Aspergillus niger. Three different species of mushrooms viz., Russla laeta, R. nobilis, and R. nigricans were collected and identified from Himalayan range of Pakistan and are found as new records for this country. Major objective of this study was to highlight the importance of these wild creatures as antifungal agents against A. niger. For this purpose methanolic extract of selected mushrooms of different concentration levels viz., 1, 1.5, 2 and 3% were used. This activity is also first time reported from Pakistan by using this group of mushrooms. Results showed that all tested mushrooms exhibit growth inhibition of A. niger and can be used as biocontrol agents. R. nigricans showed maximum inhibition of fungus growth that is 62% at 3% concentrations while minimum inhibition was observed in R. nobilis at same concentration that is 43.6%. Introduction Many people in Pakistan depend on agriculture but various crops are contaminated by phytopathogenic fungi (i.e., Aspergillus, Fusarium, Penicillium) during pre and post-harvesting processes. -

Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest
Asian Journal of Biology 9(2): 19-32, 2020; Article no.AJOB.55647 ISSN: 2456-7124 Diversity, Distribution and Morphological Characterization of Wild Macro Fungi from Gajni Forest D. R. B. Sonchita1, F. M. Aminuzzaman1*, A. A. Joty1, J. F. Tanni1, M. N. Islam1 and M. Rahaman1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration among all authors. Author DRBS collected the samples and conducted the research work. Author FMA designed and supervised the research work, collected the samples, wrote and edited the manuscript. Authors AAJ and MNI collected the samples. Authors JFT and MR managed the literature searches. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJOB/2020/v9i230084 Editor(s): (1) Dr. P. Dhasarathan, Anna University, India. Reviewers: (1) Blagoy Uzunov, Sofia University “St. Kliment Ohridski”, Bulgaria. (2) Siddhant, Durgesh Nandini Degree College, India. (3) Shengrong Liu, Ningde Normal University, China. Complete Peer review History: http://www.sdiarticle4.com/review-history/55647 Received 20 March 2020 Original Research Article Accepted 27 May 2020 Published 05 June 2020 ABSTRACT Survey on macro fungi was made in Gajni forest, Sherpur, Bangladesh which is located in between 24°18' and 25°18' north latitudes and in between 89°53' and 90°91' east longitudes. It is bounded by Meghalaya state of India on the north, Mymensingh and Jamalpur districts on the south with a wide range of ecosystem. The survey was conducted on July to December, 2018 to identify and preserve wood-rot causal macro fungi for future industrial utilization. -

Department of Plant Pathology Dhaka-1207 June
OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH ARIFA AFRIN JOTY DEPARTMENT OF PLANT PATHOLOGY SHER-E-BANGLAAGRICULTURALUNIVERSITY DHAKA-1207 JUNE, 2018 OCCURRENCE, DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH BY ARIFA AFRIN JOTY REGISTRATION NO. 12-05051 A Thesis Submitted to the Faculty of Agriculture Sher-e-Bangla Agricultural University, Dhaka-1207, in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE (M.S.) IN PLANT PATHOLOGY SEMESTER: JANUARY- JUNE, 2018 Approved by: Dr. F. M. Aminuzzaman Dr. Nazneen Sultana Professor Professor Department of Plant Pathology Department of Plant Pathology Sher-e-Bangla Agricultural University Sher-e-Bangla Agricultural University Supervisor Co- Supervisor Khadija Akhter Chairman Examination Committee ACKNOWLEDGEMENTS All admiration and praises are solely to “Almighty Allah” Whose mercy absolutely enabled the author to pursue the higher study in Agriculture discipline and complete M.S. Course and research work successfully for the degree of M.S. in Plant Pathology. The author expresses her immense respect and deepest sense of gratitude and heartfelt thanks to her most reverend teacher and Supervisor, Professor Dr. F. M. Aminuzzaman, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his untiring and efficient guidance, timely instruction, valuable advices and encouragement throughout the research work and completion of this thesis. The author is grateful to her research Co-Supervisor, Prof. Dr. Nazneen Sultana, Department of Plant Pathology, Sher-e-Bangla Agricultural University, Dhaka for his guidance, keen interest, valuable advices and continuous encouragement regarding this research. The author also wishes to pay her deep respect to Prof. -
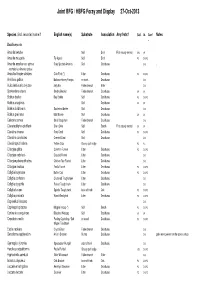
Joint BFG / HBFG Foray and Display 27-Oct-2013
Joint BFG / HBFG Foray and Display 27-Oct-2013 Species (incl. recorded name if English name(s) Substrate Association Any firsts? Coll. Id. Conf Notes . Basidiomycota Amanita betulae Soil Birch First county record SH3 SK Amanita muscaria Fly Agaric Soil Birch PC DJSPC Amanita excelsa var. spissa Grey Spotted Amanita Soil Deciduous DJS . recorded as Amanita spissa Ampulloclitocybe clavipes Club Foot (*) Litter Deciduous PC DJSPC . Armillaria gallica Bulbous Honey Fungus on roots Deciduous DJS Auricularia auricula-judae Jelly Ear Fallen branch Elder DJS Bjerkandera adusta Smoky Bracket Fallen branch Deciduous SK SK Boletus badius Bay Bolete Soil Deciduous PC DJSPC Boletus cisalpinus Soil Deciduous SK SK Boletus luridiformis Scarletina Bolete Soil Deciduous DJS Boletus pruinatus Matt Bolete Soil Deciduous SK SK Calocera cornea Small Stagshorn Fallen branch Deciduous DJS Clavariadelphus pistillaris Giant Club Soil Beech First county record SK SK Clavulina cinerea Grey Coral Soil Deciduous PC DJSPC Clavulina coralloides Crested Coral Soil Deciduous DJS Clavulinopsis helvola Yellow Club Grassy path edge PC PC Clitocybe gibba Common Funnel Litter Deciduous PC DJSPC Clitocybe nebularis Clouded Funnel Litter Deciduous DJS Clitocybe phaeophthalma Chicken Run Funnel Litter Deciduous DJS Clitocybe rivulosa Fool's Funnel Litter Deciduous PC DJSPC Collybia butyracea Butter Cap Litter Deciduous PC DJSPC Collybia confluens Clustered Toughshank Litter Deciduous DJS Collybia dryophila Russet Toughshank Litter Deciduous DJS Collybia fusipes Spindle Toughshank -

Tarset and Greystead Biological Records
Tarset and Greystead Biological Records published by the Tarset Archive Group 2015 Foreword Tarset Archive Group is delighted to be able to present this consolidation of biological records held, for easy reference by anyone interested in our part of Northumberland. It is a parallel publication to the Archaeological and Historical Sites Atlas we first published in 2006, and the more recent Gazeteer which both augments the Atlas and catalogues each site in greater detail. Both sets of data are also being mapped onto GIS. We would like to thank everyone who has helped with and supported this project - in particular Neville Geddes, Planning and Environment manager, North England Forestry Commission, for his invaluable advice and generous guidance with the GIS mapping, as well as for giving us information about the archaeological sites in the forested areas for our Atlas revisions; Northumberland National Park and Tarset 2050 CIC for their all-important funding support, and of course Bill Burlton, who after years of sharing his expertise on our wildflower and tree projects and validating our work, agreed to take this commission and pull everything together, obtaining the use of ERIC’s data from which to select the records relevant to Tarset and Greystead. Even as we write we are aware that new records are being collected and sites confirmed, and that it is in the nature of these publications that they are out of date by the time you read them. But there is also value in taking snapshots of what is known at a particular point in time, without which we have no way of measuring change or recognising the hugely rich biodiversity of where we are fortunate enough to live. -

Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka
Journal of Advances in Biology & Biotechnology 11(4): 1-18, 2017; Article no.JABB.30970 ISSN: 2394-1081 SCIENCEDOMAIN international www.sciencedomain.org Morphological Characterization of Macro Fungi Associated with Forest Tree of National Botanical Garden, Dhaka H. Rubina1, F. M. Aminuzzaman1*, M. S. M. Chowdhury1 and K. Das1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration between all authors. Author HR wrote the protocol, carried out research and wrote the first draft of the manuscript. Author KD carried out microscopic work and spore morphology. Author FMA designed and supervised the research, identified the fungal genera and also edited the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/JABB/2017/30970 Editor(s): (1) Mohammad Arif, Department of Plant Pathology, Kansas State University, Manhattan, Kansas, USA. Reviewers: (1) Chitta Ranjan Deb, Nagaland University, Lumami, India. (2) Rajesh Kumar, Rain Forest Research Institute, Assam, India. Complete Peer review History: http://www.sciencedomain.org/review-history/17933 Received 12th December 2016 th Original Research Article Accepted 6 February 2017 Published 23rd February 2017 ABSTRACT This investigation was conducted in National Botanical Garden, Dhaka located at 24°00′ N (Latitude), 90°00′ E (Longitude) to document the morphology, diversity and distribution of macro fungi during the rainy seasons of July to October, 2015. A total of 23 macro fungi samples were collected and identified to 20 species under 10 genera and 10 families. The predominant genera were Ganoderma sp., Lepiota sp., Daedeleopsis sp., Russula sp., Psythyrella sp., Lycoperdon sp., Crepidotus sp., Psilocybe sp, Flammulina sp. -

Biodiversity and Morphological Characterization of Mushrooms at the Tropical Moist Deciduous Forest Region of Bangladesh
American Journal of Experimental Agriculture 8(4): 235-252, 2015, Article no.AJEA.2015.167 ISSN: 2231-0606 SCIENCEDOMAIN international www.sciencedomain.org Biodiversity and Morphological Characterization of Mushrooms at the Tropical Moist Deciduous Forest Region of Bangladesh M. I. Rumainul1, F. M. Aminuzzaman1* and M. S. M. Chowdhury1 1Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e-Bangla Nagar, Dhaka-1207, Bangladesh. Authors’ contributions This work was carried out in collaboration with all authors. Author MIR carried out the research and wrote the first draft of the manuscript. Author FMA designed use supervised and edited the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/AJEA/2015/17301 Editor(s): (1) Sławomir Borek, Department of Plant Physiology, Adam Mickiewicz University, Poland. Reviewers: (1) Anonymous, Ghana. (2) Anonymous, India. (3) Eduardo Bernardi, Departamento de Microbiologia, Universidade Federal de Pelotas, Brazil. Complete Peer review History: http://www.sciencedomain.org/review-history.php?iid=1078&id=2&aid=9182 Received 7th March 2015 th Original Research Article Accepted 17 April 2015 Published 8th May 2015 ABSTRACT Mushroom flora is an important component of the ecosystem and their biodiversity study has been largely neglected and not documented for the tropical moist deciduous forest regions of Bangladesh. This investigation was conducted in seven different areas of tropical moist deciduous forest region of Bangladesh namely Dhaka, Gazipur, Bogra, Rajshahi, Pabna, Jaipurhat and Dinajpur. Mushroom flora associated with these forest regions were collected, photographed and preserved. A total of fifty samples were collected and identified to fourteen genera and twenty four species. -

The Diversity of Fungi in Four Irish Forest Types by Richard O'hanlon B.Sc
The diversity of fungi in four Irish forest types By Richard O’Hanlon B.Sc. (Ed) A thesis submitted for the degree of Doctor of Philosophy, At the Faculty of Science and Engineering, University of Limerick, Ireland. Supervisor: Dr Thomas Harrington, Department of Life Sciences, University of Limerick. Submitted to the University of Limerick: May 2011 i ii “The task of an ecologist” There is an old story about a man who, returning home one night found his neighbour searching the ground beneath a street lamp. “Can I help you find something?” he asked. “I lost my key” replied the neighbour. “Do you know about where you dropped it?”, “Yes” replied the neighbour “over there” pointing to a dark corner of the street. “If you dropped it over there then why are you looking here” asked the man. “Because this is where the light is” replied the neighbour. The task of the ecologist is not to bring the search to where the light is, but to bring the light to where the search is. Perry et al. (2008) iii iv Abstract Sampling of the macrofungal sporocarps, ectomycorrhizal morphotypes and vascular plants was carried out in 28 plots from four forest types (ash, oak, Scot’s pine, Sitka spruce) between the years 2007 and 2009. A total of 409 macrofungal species, 51 ectomycorrhizal morphotypes and 68 vascular plant species were recorded over the three years. It was found that at equal sampling intensities, there were no significant differences in total macrofungal species or ectomycorrhizal morphotype richness between the oak, Scot’s pine and Sitka spruce forest types. -

Suillus Lakei, an Interesting Record for Turkish Mycobiota
MANTAR DERGİSİ/The Journal of Fungus Ekim(2018)9(2)110-116 Geliş(Recevied) :12/05/2018 Research Article Kabul(Accepted) :12/06/2018 Doi:10.30708/mantar.423138 Suillus lakei, An Interesting Record For Turkish Mycobiota Ilgaz AKATA*1, Hasan Hüseyin DOĞAN2, Öyküm ÖZTÜRK3, Fuat BOZOK4 *Corresponding author: [email protected] 1Ankara University, Faculty of Science, Department of Biology, Ankara, Turkey 2SelçukUniversity, Faculty of Science, Department of Biology, Konya, Turkey 3Hacettepe University, Faculty of Science, Department of Biology, Ankara, Turkey 4Osmaniye Korkut Ata University, Faculty of Science, Department of Biology, Osmaniye, Turkey Abstract: In this study, Suillus lakei (Murrill) A.H. Sm. & Thiers) was reported for the first time from Turkey. This species is characterized by its ectomycorrhizal features and the occurrence under Pseudotsuga menziesii (Douglas fir). Besides conventional identification methods, molecular methods (ITS rDNA) were also used and results were uploaded to GenBank. According to the Genbank results, our species shows 99% similarity to other data related to Suillus lakei. A short description with molecular analysis were given in the text and the results discussed briefly. Key words: Suillus lakei, Douglas fir, ITs, new record, Turkey Suillus lakei, Türkiye Mikobiyotası İçin İlginç Bir Kayıt Öz: Bu çalışmada, Suillus lakei (Murrill) A.H. Sm. & Thiers Türkiye’den ilk defa rapor edilmiştir. Bu tür, ektomikorhizal özellikleri ve Pseudotsuga menziesii (Douglas göknarı) altında yayılış göstermesi ile karakterize edilir. Geleneksel tanımlama yöntemlerinin yanı sıra moleküler yöntemler de (ITS rDNA) kullanılmış ve sonuçlar GenBank'a yüklenmiştir. Genbank sonuçlarına göre, örneklerimiz Suillus lakei ile ilgili diğer verilere %99 benzerlik göstermektedir. Metinde moleküler analizlerle birlikte kısa bir tanımlama verilmiş ve sonuçlar kısaca tartışılmıştır. -

Volume-9, Issue-2, 2019/20 ISSN 2091-2854
INTERNATIONAL JOURNAL OF ENVIRONMENT Volume-9, Issue-2, 2019/20 ISSN 2091-2854 Received: 24 May 2020 Revised: 11 September 2020 Accepted: 2 November 2020 DIVERSITY, DISTRIBUTION AND MORPHOLOGY OF WILD MUSHROOMS COLLECTED FROM GAJNI FOREST OF BANGLADESH Arifa Afrin Joty, F. M. Aminuzzaman* , Nazneen Sultana, Akter Tanjina, Debosri Rani Biswas Sonchita and Md. Nurul Islam Department of Plant Pathology, Faculty of Agriculture, Sher-e-Bangla Agricultural University, Sher-e- Bangla Nagar, Dhaka-1207, Bangladesh *Corresponding author: [email protected] Abstract A survey was carried out in Gajni forest from June to August of 2017 and 2018 to document the diversity, distribution and morphological characterization of wild mushrooms. A total of 32 mushroom samples were collected and identified to 28 species belonging to 11 genera, under 8 families. Ganoderma sp. was found abundantly in the survey area among the other collected species and it exhibited the maximum frequency of occurrence (75%), whereas the maximum density (20.50%) was recorded for Agaricus bitorquis and the dominant host was Shal tree (Shorea robusta). The dominant genera were Ganoderma, Agaricus, Trametes, Volvariella and Amanita. The dominant family of collected wild mushrooms was Ganodermataceae followed by Polyporaceae, Agaricaceae, Amanitaceae, Rusullaceae, Pluteaceae, Marasmiaceae and Strophariaceae. Among collected species, 5 species were found edible, 12 species had medicinal value and 11 species were inedible, poisonous or of unknown importance. The specimens were deposited to the Sher- e-Bangla Agricultural University Herbarium of Macro Fungi (SHMF). This is a report of wild mushrooms diversity and their distribution in the Gajni forest region of Bangladesh. This study was asserted that a wide range of mushroom plays an important role in the ecosystem of Gajni forest and might be useful in food and industry sector in future.