Determination of Biologically Active Compounds in Hungarian Wines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1.2 Weingartenflächen Und Flächenanteile Der Rebsorten EN
1. Vineyard areas and areas under vine by grape variety Austrian Wine statistics report 1.2 Vineyard areas and areas under vine by grape variety 2 The data in this section is based on the 2015 Survey of Area under Vine, as well as feedback from the wine-producing federal states of Niederösterreich (Lower Austria), Burgenland, Steiermark (Styria) and Wien (Vienna). The main source of data for the 2015 Survey of Area under Vine was the Wein-ONLINE system operated by the Federal Ministry of Agriculture, Forestry, Environment and Water Management (BMNT). Data from the remaining federal states was collected by means of a questionnaire (primary data collection). Information relating to the (approved) nurseries was provided by the Burgenland and Lower Austrian Chambers of Agriculture and the Styrian state government (Agricultural Research Centre). According to the 2015 Survey of Area under Vine, Austria’s vineyards occupied 45,574 hectares. The planted vineyard area was 45,439 ha, which corresponds to 94 ha less (or a 0.2% decrease) in comparison to the 2009 Survey of Area under Vine. The long-running trend that suggested a shift away from white wine and towards red was quashed by the 2015 Survey of Area under Vine. While the white wine vineyard area increased by 2.3% to 30,502 ha compared to 2009, the red wine area decreased by 4.9% to 14,937 ha. Figure 1 shows the evolution of Austrian viticulture after the Second World War. The largest area under vine was recorded in 1980 at 59,432 ha. From 1980 onwards, the white wine vineyard area has continuously decreased, while the red wine vineyard area has expanded. -
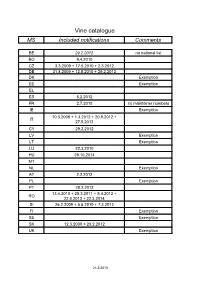
Vine Catalogue MS Included Notifications Comments
Vine catalogue MS Included notifications Comments BE 29.2.2012 no national list BG 9.4.2010 CZ 3.3.2009 + 17.5.2010 + 2.3.2012 DE 31.8.2009 + 12.5.2010 + 29.2.2012 DK Exemption EE Exemption EL ES 8.2.2012 FR 2.7.2010 no maintainer numbers IE Exemption 10.3.2008 + 1.3.2012 + 20.9.2012 + IT 27.5.2013 CY 29.2.2012 LV Exemption LT Exemption LU 22.3.2010 HU 29.10.2014 MT NL Exemption AT 2.2.2012 PL Exemption PT 28.2.2012 13.4.2010 + 25.3.2011 + 5.4.2012 + RO 22.4.2013 + 22.3.2014 SI 26.2.2009 + 5.5.2010 + 7.3.2012 FI Exemption SE Exemption SK 12.3.2009 + 20.2.2012 UK Exemption 21.5.2015 Common catalogue of varieties of vine 1 2 3 4 5 Known synonyms Variety Clone Maintainer Observations in other MS A Abbuoto N. IT 1 B, wine, pas de Abondant B FR matériel certifiable Abouriou B FR B, wine 603, 604 FR B, wine Abrusco N. IT 15 Accent 1 Gm DE 771 N Acolon CZ 1160 N We 725 DE 765 B, table, pas de Admirable de Courtiller B FR matériel certifiable Afuz Ali = Regina Agiorgitiko CY 163 wine, black Aglianico del vulture N. I – VCR 11, I – VCR 14 IT 2 I - Unimi-vitis-AGV VV401, I - Unimi-vitis- IT 33 AGV VV404 I – VCR 7, I – VCR 2, I – Glianica, Glianico, Aglianico N. VCR 13, I – VCR 23, I – IT 2 wine VCR 111, I – VCR 106, I Ellanico, Ellenico – VCR 109, I – VCR 103 I - AV 02, I - AV 05, I - AV 09, I - BN 2.09.014, IT 31 wine I - BN 2.09.025 I - Unimi-vitis-AGT VV411, I - Unimi-vitis- IT 33 wine AGTB VV421 I - Ampelos TEA 22, I - IT 60 wine Ampelos TEA 23 I - CRSA - Regione Puglia D382, I - CRSA - IT 66 wine Regione Puglia D386 Aglianicone N. -

C Ertificate 2020 Weinbau Gruber OG
Cert. No: 2-00186-2021 Weinbau Gruber OG Winzerstraße 46, 3743 Röschitz National farm No: 4284721 The above mentioned operator with the main activity "agricultural production" has been in possession of a valid inspection contract with Austria Bio Garantie GmbH since 2013-09-07. On the basis of the inspection conducted on 2020-08-12 the operator is certified according to the following rules: • Council Regulation (EC) No 834/2007 and its implementing rules, as amended • Guideline Agricultural Products from Organic Production and Derived Products, as amended This document has been issued on the basis of Article 29(1) of Regulation (EC) No 834/2007 and of Regulation (EC) No 889/2008. The declared operator has submitted his activities under control, and meets the requirements laid down in the named Regulations. The operator's products listed below may be declared as follows: Product(group): Declaration: Plant production: field bean (1,3423 ha) organic product green fallow and fallow land (5,0827 ha) conventional product vegetated not used area (0,1769 ha) conventional product grapes (1,0667 ha) plots 36, 183, 184 conventional product Grüner Veltliner Certificate 2020 Certificate grapes (1,2828 ha) plot 1 young plants without in-conversion product (1) yield Pinot Noir grapes (1,0864 ha) plot 10 young plants in-conversion product (1) without yield Rhine Riesling grapes (0,2165 ha) plot 171 in-conversion product (1) Müller Thurgau grapes (2,9046 ha) plots 6, 113 young plants in-conversion product (1) without yield Grüner Veltliner During the named period, this certificate retains Validity of the certificate from 2021-01-08: validity only if: Plant products: harvest 2020, not later than 2022-01-31 a) The products must be in accordance with the named regulations. -

Official Journal of the European Communities No L 214/ 1
16 . 8 . 80 Official Journal of the European Communities No L 214/ 1 I (Acts whose publication is obligatory) COMMISSION REGULATION (EEC) No 2164/80 of 8 August 1980 amending for the seventh time Regulation ( EEC) No 1608/76 laying down detailed rules for the description and presentation of wines and grape musts THE COMMISSION OF THE EUROPEAN on an additional label placed in the same field of COMMUNITIES , vision as the other mandatory information ; Having regard to the Treaty establishing the European Economic Community, Whereas the nominal volume of containers with a volume of not less than 5 ml and not more than 10 1 suitable for putting up wines and grape musts which Having regard to Council Regulation (EEC) No are the subject of intra-Community trade is governed 337/79 of 5 February 1979 on the common organi by Council Directive 75/ 106/EEC of 19 December zation of the market in wine ('), as last amended by 1974 on the approximation of the laws of the Regulation (EEC) No 1988 / 80 (2 ), and in particular Member States relating to the making-up by volume Article 54 ( 5) thereof, of certain prepackaged liquids (8 ), as amended by Directive 79/ 1005 /EEC ( 9); whereas it is necessary, Whereas Council Regulation ( EEC) No 355 /79 of first, to adjust Regulation (EEC) No 1608 /76 in line 5 February 1979 laying down general rules for the with the amendments to that Directive and , secondly, description , and presentation of wines and grape in order to enable the wines and grape musts already musts (■'), as amended by Regulation (EEC) No -

English All List
Wine Sparkling Residual Award Category Producer Wine Vintage Country Region % Variety 1 % Variety 2 Inporter or Distributor No. Sugar Cabernet 13328 DG Still Red BODEGA NORTON CABERNET SAUVIGNON RESERVA 2014 ARGENTINA MENDOZA 100 ENOTECA CO., LTD. Sauvignon Cabernet 13326 DG Still Red BODEGA NORTON GERNOT LANGES 2012 ARGENTINA MENDOZA 60 Malbec 20 ENOTECA CO., LTD. Sauvignon Cabernet VINOS YAMAZAKI CO., 13003 DG Still Red BODEGAS FABRE S.A. VINALBA RESERVA CABERNET SAUVIGNON 2015 ARGENTINA MENDOZA 100 Sauvignon LTD 13517 DG Still Red MENDOZA VINEYARDS R AND B MALBEC 2013 ARGENTINA MENDOZA 100 Malbec CENTURY TRADING CO., 13524 DG Still Red MYTHIC ESTATE MYTHIC BLOCK MALBEC 2013 ARGENTINA MENDOZA 100 Malbec LTD. 10917 DG Still White PAMPAS DEL SUR EXPRESION ARGENTINA TORRONTES 2016 ARGENTINA MENDOZA 100 Torrontes FOODEM CO., LTD. 12885 DG Still Red VINITERRA SINGLE VINEYARD 2013 ARGENTINA MENDOZA 100 Malbec TOA SHOJI CO., LTD 12270 DG Still Red BODEGA EL ESTECO DON DAVID TANNAT RESERVE 2014 ARGENTINA SALTA 100 Tannat SMILE CORP. 14059 DG Still White CHAKANA MAIPE TORRONTES 2016 ARGENTINA SALTA 100 Torrontes OVERSEAS CO., LTD. Cabernet TOKO TRADING CO., 12728 DG Still Red ELEMENTOS ELEMENTOS CABERNET SAUVIGNON 2016 ARGENTINA SALTA 100 Sauvignon LTD 10532 DG Still White BODEGAS CALLIA ALTA CHARDONNAY - TORRONTES 2016 ARGENTINA SAN JUAN 60 Chardonnay 40 Torrontes MOTTOX INC. SOUTH VINOS YAMAZAKI CO., 11578 DG Still Red 3 RINGS 3 RINGS SHIRAZ 2014 AUSTRALIA 100 Shiraz AUSTRALIA LTD Cabernet ASAHI BREWERIES, 11363 DG Still Red KATNOOK ESTATE KATNOOK ESTATE CABERNET SAUVIGNON 2012 AUSTRALIA SOUTH AUSTRALIA 100 Sauvignon LTD. ASAHI BREWERIES, 11365 DG Still White KATNOOK ESTATE KATNOOK ESTATE CHARDONNAY 2013 AUSTRALIA SOUTH AUSTRALIA 100 Chardonnay LTD. -

Catalogue of Producers 2018 January / August Visit Our Full Wine Portfolio: Catalogue of Producers 2018
Catalogue of Producers 2018 January / August Visit our full wine portfolio: Cataloguewww.winehungary.co.uk/portfolio of Producers 2018 Visit our full wine portfolio 1 www.winehungary.co.uk/portfolio WELCOME The Wines of Hungary UK project brings great value wines into the UK with an emphasis on quality. Launched in 2014, Wines of Hungary UK, is the key project of Wine Communications, a London based wine marketing and communications agency. We represent a group of established Hungarian winemakers with ambitions to introduce their wines to the UK market and help them in building sales. Wine Communications works on both business to business and business to consumer level. We work with wine makers and assist them through each steps of the distribution channel, either with market entry strategy into the UK market to lead generation, or driving volumes with sales support communication and promotional activities. Hungarian wines have been going from strength to strength in the UK over the last few years. According to the WSTA Annual Wine Report 2017, Hungarian wines were amongst the top 10 importers of both white and red wines to the UK in 2016, bringing in 69,498 and 35, 229 hectolitres respectively. Our aim is to build on this momentum with events like today, providing education and expertise from within the industry. The consumer demand for volcanic soils allow lovely Hungarian wines is significant. minerality, which makes them Importers are adding to particularly food friendly. This their lists and we are seeing had been helped by the more Hungarian wines in outstanding vintage that Michelin Starred and fine Hungary had in 2016, 2017, dining restaurants in London and 2018 is looking promising than ever before The wines so it’s a great opportunity to are high quality and Hungary’s join us and try the wines. -

The Wine Century Club 0
The Wine Century Club APPLICATION FOR MEMBERSHIP AT S RE EA First Name: Middle Name: G L E O H F Last Name: Email: T EST 2005 Address: T VENI VICI H E B Address 2: W U VINO L IN C E Y CENTUR City: State/Province: Zip: Country: grape count: Instructions: Check the box next to each grape variety you have tasted. For varieties not listed here, use the blank spaces at the bottom of each section. Grape varieties that you've tried only in blends with other varieties are permitted. Wine Name, Produder, Region & Vintage are optional (but required if you’re going for trebble membership or higher). If you have at least 100 varieties checked, email this form to [email protected] or upload it at www.winecentury.com/upload. Please note that the application is entirely on the honor system; should you lie, may the wrath of Bacchus curse your palate! WHITE GRAPES Wine Name, Winemaker, Region & Vintage (Optional) Airén Albariño Albarola Aligoté Arinto Arneis Arvine Asprinio Bianco Assyrtiko Auxerrois Avesso Bacchus Bellone Biancolella Bical Blanc de Morgex Bombino Bianco Bornova Misketi WHITE GRAPES Wine Name, Winemaker, Region & Vintage (Optional) Bosco Bourboulenc Bual Bukettraube Carricante Catarratto Chardonnay Chasselas Chenin Blanc Clairette Cococciola Coda di Volpe Colombard Cortese Cserzegi Fuszeres Delaware Emir Erbaluce Falanghina Favorita Feteasca Alba Fiano Folle Blanc Forastera Fruilano Furmint Garganega Gewürztraminer Godello Gouais blanc Grechetto Greco The Wine Century Club APPLICATION PAGE 2 WHITE GRAPES Wine Name, Winemaker, Region -
Austrian Food & Wine
AUSTRIAN FOOD & WINE INDEX Austria The Culinary Heart of Central Europe ...........3 BeliebteBeliebte SchmankerlSchmankerlBeliebtePopular Favourites .................................... 10 SchmankerlBeliebte SchmankerlBeliebte Beliebte Roast Pork | Meat Rice | Rissoles | Pasta Ham Bake Schmankerl Schmankerl AusAus Teichen, Teichen,From our Lakes, Rivers and Ponds ............... 16 FlüssenFlüssenAus Teichen, und und Seen Seen FlüssenAus Teichen, und SeenTrout, Char or Whitefish | Pike Dumplings in White Wine Sauce | FlüssenAus Teichen, und Seen Aus Teichen, Flüssen und SeenPaprika Pikeperch | Carp with Root Vegetables Flüssen und Seen GeflüGeflügeltegelte KöstliKöstliGeflüchkeitenchkeitengPoultryelte Delicacies ...................................... 22 KöstliGeflüchkeitengelte KöstliGeflüchkeiten gelte Roast Duck, Martini Goose | Fried Chicken with Potato Salad | Geflügelte Köstlichkeiten Köstlichkeiten Roast Chicken | Creamy Paprika Chicken with Spätzle KlassikerKlassiker vom vom KlassikerMilchkalbMilchkalb vom KlassikerMilchkalbClassic vom Favourites from Veal Meat ................ 28 KlassikerMilchkalb vom Klassiker vom Milchkalb Milchkalb Roast Loin of Veal with Kidneys | Ragout of Veal Lights | Sauteed Calf’s Liver | Wiener Schnitzel with Potato Salad DieDie Wiener Wiener RindfleischkücheRindfleischkücheDie Wiener RindfleischkücheDie Wiener RindfleischkücheDie Wiener Die Wiener RindfleischkücheTraditional Viennese Beef Dishes ................. 34 Rindfleischküche Boiled Beef | Beef Rolls | Braised Shoulder of Beef | ÖsterreichÖsterreich zur zurRoast -

Canada-EU Wine and Spirits Agreement
6.2.2004 EN Official Journal of the European Union L 35/3 AGREEMENT between the European Community and Canada on trade in wines and spirit drinks THE EUROPEAN COMMUNITY, hereafter referred to as ‘the Community', and CANADA, hereafter jointly referred to as ‘the Contracting Parties', RECOGNISING that the Contracting Parties desire to establish closer links in the wine and spirits sector, DESIROUS of creating more favourable conditions for the harmonious development of trade in wine and spirit drinks on the basis of equality and mutual benefit, HAVE AGREED AS FOLLOWS: TITLE I — ‘labelling' shall mean any tag, brand, mark, pictorial or other descriptive matter, written, printed, stencilled, marked, embossed or impressed on, or attached to, a INITIAL PROVISIONS container of wine or a spirit drink, Article 1 — ‘WTO Agreement' refers to the Marrakesh Agreement establishing the World Trade Organisation, Objectives 1. The Contracting Parties shall, on the basis of — ‘TRIPs Agreement' refers to the Agreement on trade-related non-discrimination and reciprocity, facilitate and promote aspects of intellectual property rights, which is contained trade in wines and spirit drinks produced in Canada and the in Annex 1C to the WTO Agreement, Community, on the conditions provided for in this Agreement. 2. The Contracting Parties shall take all reasonable measures — ‘1989 Agreement' refers to the Agreement between the to ensure that the obligations laid down in this Agreement are European Economic Community and Canada concerning fulfilled and that the objectives set out in this Agreement are trade and commerce in alcoholic beverages concluded on attained. 28 February 1989. Article 2 2. -

Weinviertel DAC Grüner Veltliner Sauvignon Blanc Riesling R
Sortimentliste/ Bestellschein / Reservierung 2021 2020 * 12% vol, trocken Weinviertel DAC 2020 * 12,5 %vol Rosecco- Frizzante ...Rosi´s prickelnder Genuss aus dem Zweigelt-Rosé. schönes grün, herrliche Fruchtigkeit in der Nase & pfeffrige Ein Frizzante mit angenehmen Beerennoten machen diesen besonderes Anklänge nach weißen Pfeffer, Zitrusaromen runden den Duft ab schmackhaft, harmonisches Fruchtspiel am Gaumen, pickelnder und spiegelt sich wunderbar am Gaumen wider - mit dem Abgang deshalb ist er ein perfekter Start Begleiter bei vielen Anlässen. gewissen Pfefferl ist er ein perfekter Speisenbegleiter der österreichischen Küche. Ein Weinviertler Veltliner mit Typizität & Ausdruck. Zweigelt “Classic” 2018 * 12,5%vol dunkles Rubinrot, köstliche Beerenfrucht erinnert an reife Kirschen Winzerin” 2020* 12,5 %vol und schwarze Johannisbeere, die sich am Gaumen widerspiegeln und Grüner Veltliner “ schönes Gelb, zartblumige Würze, schöne Frucht leicht exotisch, vollmundig wirken, sehr harmonisch & ansprechend mit einer guten fein geprägt am Gaumen, balanciert & angenehm zu trinken, Tanninstruktur! mit einer animierenden Säurestruktur. Die Winzerin Rosemarie: Ein frech- fruchtiger Wein wie ich ihn mag. Rösler 2018 * 13%vol (Neuzüchtete Piwisorte aus Zweigelt x Blaufränkisch) schönes tiefdunkles Rot, schokoladeartige Nuancen mit vielen Sauvignon blanc 2020 * 13 %vol fruchtigen Beerennoten, stoffig & samtig am Gaumen mit einer guten angenehmer Paprika & Holunderblütenduft in der Nase, Körperfülle, ein moderner neuer Wein für besondere zarte grasige Noten nach Wiesenkräuter am Gaumen, eleganter Rotweinliebhaber! Körper, saftig & feingliedrig, besonderes Säurespiel lädt zum Restbestand nächsten Schluck ein. Ein sympathischer Wein, der einen netten Abend perfekt umrahmt. Blauburger “Classic” 2016 * 13%vol leuchtendes Granatrot, schöne Nase nach fruchtigen Herzkirschen, samtig, geschmeidige Waldbeerfrucht am Gaumen, mit einer weichen Riesling Classic” 2020 * 12,5 %vol “ - samtigen Tanninfülle. -

Kürzelliste Für Weinformulare
Kürzelliste für Weinformulare Stand: 20.04.2021 Art Bezeichnung Kürzel Art Bezeichnung Kürzel Art Eiswein EIW Geläger GE Industriewein INW Konzentrierter KM Kabinett KAB Traubenmost Landwein LDW Maische MA Qualitätswein QUW Most MS Ruster Ausbruch RAB Rektifiziertes RK Sonstige Erzeugnisse SOE Traubenmostkonzentrat Spätlese SPL (RTK) Strohwein STW Sonstiges Erzeugnis SE Sturm für Qualitätsstufe STU Sturm für Art ST Teilweise gegorener TGT Trauben TB Traubenmost Wein WE Trockenbeerenauslese TBA Herkunft Wein (mit Sorte und RSW Ausland AUSL Jahrgang) Bergland BLXX Wein (ohne Rebsorte WEI Burgenland WLBL und Jahrgang) Carnuntum WLCA Rebsortenwein Eisenberg WLEB Bronner BN Kamptal WLKA Cabernet blanc CB Kärnten BLKA Cabernet Jura CJ Kremstal WLKT Donauriesling DR Leithaberg WLLB Donauveltliner DV Mittelburgenland WLMB Johanniter JO Neusiedlersee WLNS Pinot Nova PI Niederösterreich WLNO Regent RE Oberösterreich BLOO Sorte für Direktträgerwein (SOE / WEI) Österreich OEST Direktträger DT Rosalia WLRO Sorte für LDW, QUW u. PRW Salzburg BLSB Blauburger BL Steiermark SLST Blauer Burgunder (Blauer BB Steirerland SLXX Spätburgunder, Südsteiermark SLSS Blauburgunder, Pinot Noir) Thermenregion WLTH Blauer Portugieser BP Tirol BLTI Blauer Wildbacher BW Traisental WLTT Blaufränkisch (Frankovka) BF Verschnitt von mehreren EUXX Weinen der EU Blütenmuskateller BM Vorarlberg BLVO Bouvier BO Vulkanland Steiermark SLVL Cabernet Franc CF Wachau WLWA Cabernet Sauvignon CS Wagram WLWG Chardonnay (Morillon) CH Weinland WLXX Frühroter Veltliner FV (Malvasier) Weinviertel -

European Project Grapegen 06 - Grapevine Genetic Resources - Version 21 January 2011 P
European Project GrapeGen 06 - Grapevine Genetic Resources European Grapevine Catalogue: Towards a Comprehensive List T. Lacombe, L. Audeguin, M. Boselli, B. Bucchetti, F. Cabello, M. Crespan, C. D’Onofrio, J. Eiras Dias, S. Ercisli, M. Gardiman, MS. Grando, S. Imazio, O. Jandurova, A. Jung, E. Kiss, P. Kozma, E. Maul, D. Maghradze, C. Martinez, G. Muñoz, J-K. Pátková, I. Pejic, E. Peterlunger, D. Pitsoli, D. Preiner, S. Raimondi, F. Regner, G. Savin, S. Savvides, A. Schneider, J-L. Spring, A. Szoke, A. Veres, J-M. Boursiquot, R. Bacilieri and P. This Annex 3 B : Official national catalogues of grapevine varieties for Member States of the European Union and the Third Countries partner of the GrapeGen 06 Project Legend : before the arrows, name of the variety as registered in the country . After the arrows, common prime name of the variety according to VIVC database when referenced, # identification number of the variety, species of the variety, sex (H = hermaphrodite, F = female, M = male), colour of berry skin (B = yellow-green, N = blue-black, Rg = red, Rs = rose, G = grey). Austria AUT National Catalogue version 2008 Alphonse-Lavalle (AUT) >>> ALPHONSE LAVALLEE # 349 - vinifera - H - N Angela (AUT) >>> ANGELA # 20342 - interspecific cross - H - B Aron (AUT) >>> ARON # 14014 - interspecific cross - - B Attica (AUT) >>> ATTIKA SEEDLESS # 17309 - vinifera - - Rg Attila (AUT) >>> ATTILA # 756 - vinifera - - B Bacchus (AUT) >>> BACCHUS WEISS # 851 - - H - B Bianca (AUT) >>> BIANCA # 1321 - interspecific cross - H - B Birstaler Muskat (AUT)