Compound Name
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 31/04 (2006.01) B01J 31/18 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 31/14 (2006.01) B01J 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 12/065285 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 November 2012 (15.1 1.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/323,328 12 December 201 1 (12. 12.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): CHEV¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, RON PHILLIPS CHEMICAL COMPANY LP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/US]; 10001 Six Pines Drive, The Woodlands, Texas TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 77380 (US). -

Attachment 3-1 Guidance for Developing Ecological Soil
Attachment 3-1 Guidance for Developing Ecological Soil Screening Levels (Eco-SSLs) Eco-SSL Standard Operating Procedure (SOP # 1): Plant and Soil Invertebrate Literature Search and Acquisition OSWER Directive 92857-55 November 2003 This page intentionally left blank OVERVIEW Currently, there is a lack of clear guidance in setting terrestrial effect thresholds when conducting risk assessments. Without an EPA-approved, peer-reviewed, ecologically-based terrestrial effect database, the process to develop thresholds is problematic both to EPA, other federal agencies, states, and concerned private parties. Identification of published toxicity studies on invertebrates, microbial processes and plants is a key step in the derivation of benchmarks. The purpose of the Task Group 4, Standard Operating Procedure Number 1: Literature Search and Acquisition (referred to as TG4-SOP#1) is to document procedures used to identify and acquire potentially relevant toxicology literature for use in setting ecological soil screening levels. The literature search strategy is designed to locate worldwide terrestrial toxicity literature that includes the effects of chemicals of concern on terrestrial soil-dwelling invertebrates and plants. The literature acquisition process is designed to ensure timely acquisition of relevant publications. LITERATURE IDENTIFICATION Potentially relevant literature for developing ecological soil screening levels (Eco-SSLs) is identified by examining hard copies of relevant journals, bibliographies and guidance publications and through the use of a comprehensive computerized literature search strategy. These procedures are designed to locate worldwide terrestrial toxicology literature that includes the effects of specific toxic substances with an emphasis on exposure via soil. Paper-based Literature Identification The paper-based literature identification process includes the scanning of relevant review article bibliographies and key journals held in the U.S. -

AP Chemistry Summer Assignment 2021-22 School Year
AP Chemistry AP Chemistry Summer Assignment 2021-22 School Year Chemical Foundations Chapter 1 1. How many significant figures are in the following numbers? a. 0.00150 b. 0.1205 c. 200 d. 2.00 X 10 3 2. Complete the operation and report using the correct number of significant figures a. 26.20 - 0.5= b. 2.5 + 3.25 = c. .040 X 2.0 = d. 3.25 / 4 = e. 3.0 X 10 -3 X 6 = Atomic , Molecules Ions CH 2 and Stoichiometry CH 3 with Periodicity CH 7 3. What is an isotope? Refer to the isotope of Uranium 92U 4. How many protons and neutrons are in the nucleus of this isotope. 5. How many electrons are in a single atom of Uranium 6. What is the mass of this isotope of Uranium. 7. Assume silicon has three major isotopes in nature. The average atomic mass of silicon is 28.09 amu. Fill in the missing information in the table. Isotope Mass (amu) Abundance 28Si 27.89 29Si 4.70% 30Si 29.97 3.09% 8. Which color of light has the highest frequency, red or green ? 9. Which color of light has the longest wavelength, green or violet? 10. Hydrogen emits light with a wavelength of 410 nm, what is the frequency of this light? 11. What is the electron configuration, orbital notation and noble gas notation for phosphorus? For bromine? How many unpaired electrons does phosphorus have? How many unpaired electrons does bromine have? 12. What is the charge for a phosphorus ion? Why does it make this charge? 13. -
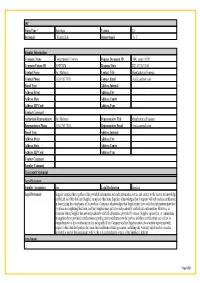
Material Composition Declaration
IPC Form Type * Distribute Version 2.0 Sectionals - MaterialInfo Subsectionals - A- C Supplier Information Company Name Contemporary Controls Request Document ID EISK_series_MCD Company Unique ID 363857474 Response Date 2021-07-30-12:00 Contact Name Neil Maloney Contact Title Manufacturing Engineer Contact Phone (630) 963 7070; Contact Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Authorized Representative Neil Maloney Representative Title Manufacturing Engineer Representative Phone (630) 963 7070; Representative Email [email protected] Email Type Address Internal Address Street Address City Address State Address Contry Address ZIP Code Address Type Contact Comment Supplier Comment Uncertainty Statement Legal Statement Supplier Acceptance true Legal Declaration Standard Legal Statement Supplier certifies that it gathered the provided information and such information is true and correct to the best of its knowledge and belief, as of the date that Supplier completes this form. Supplier acknowledges that Company will rely on this certification in determining the compliance of its products. Company acknowledges that Supplier may have relied on information provided by others in completing this form, and that Supplier may not have independently verified such information. However, in situations where Supplier has not independently verified information provided by others, Supplier agrees that, at a minimum, its suppliers have provided certifications regarding their contributions to the part(s), and those certifications are at least as comprehensive as the certification in this paragraph. If the Company and the Supplier enter into a written agreement with respect to the identified part(s), the terms and conditions of that agreement, including any warranty rights and/or remedies provided as part of that agreement, will be the sole and exclusive source of the Supplier’s liability Attachment . -

The True Formula
ON THE TRUE FORMULA FOR PERMANGANATES. DISSERTATION PRESENTED TO THE FACULTY OF THE UNIVERSITT7 ' OF VIRGINIA. In application fir the Degree of Dadar of 'Pflilawplzj'. BY CHARLES M. B‘RADBURY, 0F VIRGINIA. THESIS —Permanga;zz}: acid is moiwéaszc, and the per-:V _ mangamztes are represented 6}! the generalfbmwla R (Mn 04 p. -' : . ; V To His Fat/tor, 31m ‘18. granary, (35511. 77:13 Paper is Inscribed IN TOKEN OF AFFECTION, BY film 9mm, Preface. The greater part of the following paper was written nearly a year ago. As it was nearing completion, I found, from an allusion in .an article by M. Raoult, of Paris, (see Bzzlleiz'n dc Soc. C/u'm. de Paris, XLVI, p. 805), that my conclusion as to the true formula for permanganates had been reached already by that eminent chemist, by means of a new method, origi- nated by himself, for determining molecular weights. Upon examining M. Raoult‘s original papem‘fHéWIz—Iifilc C/zz'm. ct dc Ply/5., (6), VIII, July, 1886, p. 3 30, however, it was found that nothing was given concerning permanganates, beyond the simple statement that they were shown by the method to be monobasic. T Dr. Victor Meyer and Dr. Auwers, of Gottingen, have recently extended and simplified the method of M. Raoult, (see Berk/zit a’n’ Dads. Chem. 656115., for February 27 and March 12, 1888), and I am therefore enabled to add materially to the evidence already collected in the dissertation, by a con- vincing application of this method. ‘ Had the detailed results of M. -

Studies on the Behavior of Spontaneous Reductive Decomposition of Hydrogen Permanganate in Water for HP/CORD Process
Studies on the Behavior of Spontaneous Reductive Decomposition of Hydrogen Permanganate in Water for HP/CORD Process Hyun-Kyu Lee*, June-Hyun Kim, Won-Zin Oh, and Sang-June Choi Kyungpook National University, 80 Daehakro, Bukgu, Daegu 41566, Republic of Korea *[email protected] 1. Introduction (Optima 2100DV, PerkinElmer Co., USA), respectively, for determining whether the cation + + The permanganic acid (HMnO4) as oxidizing agent exchange (K ˧H ) was completely conducted. is usually used to dissolve the NPP oxide layers containing Cr (III) in HP/CORD process [1]. 2.3 Apparatus and procedure However, little attention has been made for the spontaneous reductive decomposition of HMnO4, All experiments were carried out in 500 mL glass which is a competing reaction with the oxidation of reaction vessels equipped with reflux condenser, the chromium oxide by permanganate. The objective thermometer, mechanical stirrer, and electric heating of this study is to investigate the spontaneous mantle. Reaction volumes were nomally 200 mL and reductive decomposition behavior of HMnO4 for were stirred at 250 rpm. The temperature was HP/CORD process, depending on the variation of maintained at 90°C. Aliquots obtained from each initial concentration of HMnO4. experiment were quickly cooled, diluted, and placed in a dark bottle to avoid further reaction. The concentration of residual permanganate was 2. Materials and methods determined by UV-visible spectrophotometer (Agilent 8453, Agilent technologies, USA) after 2.1 Reagents filtration (0.2 um, Whatman, nylon membrane filters). All reagents were analytical grade and were used 3. Results and discussion without further purification. Deionized water (18.3 Mȍācm), which was prepared using a water 3.1 Effect of the initial concentration of HMnO purification system (Nex Power 1000, Human 4 corporation, Korea) was used to prepare all aqueous The change of concentration of residual solutions. -

Common Ions and Their Charges
Common Ions and Their Charges A mastery of the common ions, their formulas and their charges, is essential to success in AP Chemistry. You are expected to know all of these ions on the first day of class, when I will give you a quiz on them. You will always be allowed a periodic table, which makes indentifying the ions on the left “automatic.” For tips on learning these ions, see the opposite side of this page. From the table: Ions to Memorize Cations Name Cations Name H+ Hydrogen Ag+ Silver Li+ Lithium Zn2+ Zinc + 2+ Na Sodium Hg2 Mercury(I) + + K Potassium NH4 Ammonium Rb+ Rubidium Cs+ Cesium Be2+ Beryllium Anions Name 2+ - Mg Magnesium NO2 Nitrite 2+ - Ca Calcium NO3 Nitrate 2+ 2- Ba Barium SO3 Sulfite 2+ 2- Sr Strontium SO4 Sulfate 3+ - Al Aluminum HSO4 Hydrogen sulfate (bisulfate) OH- Hydroxide Anions Name CN- Cyanide - 3- H Hydride PO4 Phosphate - 2- F Fluoride HPO4 Hydrogen phosphate - - Cl Chloride H2PO4 Dihydrogen phosphate Br- Bromide NCS- Thiocyanate - 2- I Iodide CO3 Carbonate 2- - O Oxide HCO3 Hydrogen carbonate (bicarbonate) S2- Sulfide ClO- Hypochlorite 2- - Se Selenide ClO2 Chlorite 3- - N Nitride ClO3 Chlorate 3- - P Phosphide ClO4 Perchlorate As3- Arsenide BrO- Hypobromite - Type II Cations Name BrO2 Bromite 3+ - Fe Iron(III) BrO3 Bromate 2+ - Fe Iron(II) BrO4 Perbromate Cu2+ Copper(II) IO- Hypoiodite + - Cu Copper(I) IO2 iodite 3+ - Co Cobalt(III) IO3 iodate 2+ - Co Cobalt(II) IO4 Periodate 4+ - Sn Tin(IV) C2H3O2 Acetate 2+ - Sn Tin(II) MnO4 Permanganate 4+ 2- Pb Lead(IV) Cr2O7 Dichromate 2+ 2- Pb Lead(II) CrO4 Chromate 2+ 2- Hg Mercury(II) O2 Peroxide 2- C2O4 Oxalate - NH2 Amide 3- BO3 Borate 2- S2O3 Thiosulfate Tips for Learning the Ions “From the Table” These are ions can be organized into two groups. -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

PERMANGANATE OXIDATIONS in NON-AQUEOUS SOLVENTS* By
PERMANGANATE OXIDATIONS IN NON-AQUEOUS SOLVENTS* I. OXIDATION BY TRKPHENYLMETHYLARSONIUM PERMANGANATE By N. A. GIBSON^ and J. W. HOSKING? Quaternary arsonium cations have been widely used in analysis,l mainly in the formation, with anionic complexes, of ion-association compounds which are soluble in aprotic solvents.2 The triphenylmethylarsonium cation has been used to form ion-association compounds with anionic complexes of a number of metals3-' and has also been used to form ion-association compounds with the ~ermanganate,~di- chromate,g and triiodidelo ions. The tetraphenyIarsonium cation has been used to extract the permanganate ion into non-aqueous solvents.llJ2 The permanganate ion can be extracted into non-polar solvents from acid aqueous solutions containing tributylphosphate. The triphenylarsine oxide permanganic acid adduct is soluble in chloroform and nitrobenzene.13 Triphenylmethplarsonium permanganate has been isolateds and is soluble in aprotic polar solvents. It is very soluble in chloroform and nitrobenzene (saturated solutions are approximately l~ and 0.8~respectively) and is sufficiently stable in these solvents to make possible a study of oxidizing properties of the permanganate ion in non-aqueous media. While triphenylmethylarsonium dichromate has no oxidizing properties in non- aqueous solvents,ls the corresponding permanganate behaves like permanganate ion in neutral solution, being reduced in the presence of a suitable reductant to insoluble manganese dioxide. Preliminary investigation of the oxidizing properties of the permanganate ion in chloroform showed that propan-2-01 was oxidized quantitatively to acetone. The results of a typical reaction are shown in Table 1. Kinetics of this reaction and the oxidizing properties with regard to other functional groups will be reported later. -

Material Safety Data Sheet
Purafil® Chemisorbant Media MSDS Page 1 of 5 Revision Date: 07/2008 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE PREPARATION AND OF THE COMPANY Product Identification: Purafil® Chemisorbant Media Product Synonyms: Purafil® Media, Purafil® II Chemisorbant Media, Purafil® FS Chemisorbant, Purafil® Diving Life Support Media, Purasorb Media, Puralum WW Media, Permasorb® Media Use of the preparation: This product is intended for use in gas-phase air filtration. Company Identification: Purafil, Inc. 2654 Weaver Way Doraville, GA 30340 / USA Company Contact Numbers: Telephone: (770) 662-8545 Facsimile: (770) 263-6922 2. COMPOSITION Common Chemical Name Synonyms CAS Number Wt % EC Number EU Classification aluminum oxide activated aluminas; 1333-84-2* <67 (non-fibrous) activated and amorphous aluminas water dihydrogen oxide 7732-18-5 <35 231-791-2 proprietary ingredient -- -- <26 -- potassium permanganate permanganate of potash; 7722-64-7 4 - 8 231-760-3 O; R8 chameleon mineral; Xn; R22 permanganic acid, N; R50-53 potassium salt * For TSCA inventory reporting purposes, CAS No. 1344-28-1 (EC# 215-691-6) was assigned for all forms of aluminum oxide instead of the CAS No. 1333-84-2 as indicated above. Composition Comments: For the full text of R phrases mentioned in this section, see Section 16. 3. HAZARDS IDENTIFICATION Most Important Hazards: - If crushed or handled extensively, dust may evolve and can be irritating to the eyes, skin, and respiratory tract. Adverse Human Health Effects: - The following medical conditions may be aggravated by exposure to the product: asthma, chronic lung disease, and skin rashes. - If the product contacts the skin with water, it may leave a stain of insoluble products on the skin.