Studies on the Behavior of Spontaneous Reductive Decomposition of Hydrogen Permanganate in Water for HP/CORD Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The True Formula
ON THE TRUE FORMULA FOR PERMANGANATES. DISSERTATION PRESENTED TO THE FACULTY OF THE UNIVERSITT7 ' OF VIRGINIA. In application fir the Degree of Dadar of 'Pflilawplzj'. BY CHARLES M. B‘RADBURY, 0F VIRGINIA. THESIS —Permanga;zz}: acid is moiwéaszc, and the per-:V _ mangamztes are represented 6}! the generalfbmwla R (Mn 04 p. -' : . ; V To His Fat/tor, 31m ‘18. granary, (35511. 77:13 Paper is Inscribed IN TOKEN OF AFFECTION, BY film 9mm, Preface. The greater part of the following paper was written nearly a year ago. As it was nearing completion, I found, from an allusion in .an article by M. Raoult, of Paris, (see Bzzlleiz'n dc Soc. C/u'm. de Paris, XLVI, p. 805), that my conclusion as to the true formula for permanganates had been reached already by that eminent chemist, by means of a new method, origi- nated by himself, for determining molecular weights. Upon examining M. Raoult‘s original papem‘fHéWIz—Iifilc C/zz'm. ct dc Ply/5., (6), VIII, July, 1886, p. 3 30, however, it was found that nothing was given concerning permanganates, beyond the simple statement that they were shown by the method to be monobasic. T Dr. Victor Meyer and Dr. Auwers, of Gottingen, have recently extended and simplified the method of M. Raoult, (see Berk/zit a’n’ Dads. Chem. 656115., for February 27 and March 12, 1888), and I am therefore enabled to add materially to the evidence already collected in the dissertation, by a con- vincing application of this method. ‘ Had the detailed results of M. -

PERMANGANATE OXIDATIONS in NON-AQUEOUS SOLVENTS* By
PERMANGANATE OXIDATIONS IN NON-AQUEOUS SOLVENTS* I. OXIDATION BY TRKPHENYLMETHYLARSONIUM PERMANGANATE By N. A. GIBSON^ and J. W. HOSKING? Quaternary arsonium cations have been widely used in analysis,l mainly in the formation, with anionic complexes, of ion-association compounds which are soluble in aprotic solvents.2 The triphenylmethylarsonium cation has been used to form ion-association compounds with anionic complexes of a number of metals3-' and has also been used to form ion-association compounds with the ~ermanganate,~di- chromate,g and triiodidelo ions. The tetraphenyIarsonium cation has been used to extract the permanganate ion into non-aqueous solvents.llJ2 The permanganate ion can be extracted into non-polar solvents from acid aqueous solutions containing tributylphosphate. The triphenylarsine oxide permanganic acid adduct is soluble in chloroform and nitrobenzene.13 Triphenylmethplarsonium permanganate has been isolateds and is soluble in aprotic polar solvents. It is very soluble in chloroform and nitrobenzene (saturated solutions are approximately l~ and 0.8~respectively) and is sufficiently stable in these solvents to make possible a study of oxidizing properties of the permanganate ion in non-aqueous media. While triphenylmethylarsonium dichromate has no oxidizing properties in non- aqueous solvents,ls the corresponding permanganate behaves like permanganate ion in neutral solution, being reduced in the presence of a suitable reductant to insoluble manganese dioxide. Preliminary investigation of the oxidizing properties of the permanganate ion in chloroform showed that propan-2-01 was oxidized quantitatively to acetone. The results of a typical reaction are shown in Table 1. Kinetics of this reaction and the oxidizing properties with regard to other functional groups will be reported later. -

Material Safety Data Sheet
Purafil® Chemisorbant Media MSDS Page 1 of 5 Revision Date: 07/2008 MATERIAL SAFETY DATA SHEET 1. IDENTIFICATION OF THE PREPARATION AND OF THE COMPANY Product Identification: Purafil® Chemisorbant Media Product Synonyms: Purafil® Media, Purafil® II Chemisorbant Media, Purafil® FS Chemisorbant, Purafil® Diving Life Support Media, Purasorb Media, Puralum WW Media, Permasorb® Media Use of the preparation: This product is intended for use in gas-phase air filtration. Company Identification: Purafil, Inc. 2654 Weaver Way Doraville, GA 30340 / USA Company Contact Numbers: Telephone: (770) 662-8545 Facsimile: (770) 263-6922 2. COMPOSITION Common Chemical Name Synonyms CAS Number Wt % EC Number EU Classification aluminum oxide activated aluminas; 1333-84-2* <67 (non-fibrous) activated and amorphous aluminas water dihydrogen oxide 7732-18-5 <35 231-791-2 proprietary ingredient -- -- <26 -- potassium permanganate permanganate of potash; 7722-64-7 4 - 8 231-760-3 O; R8 chameleon mineral; Xn; R22 permanganic acid, N; R50-53 potassium salt * For TSCA inventory reporting purposes, CAS No. 1344-28-1 (EC# 215-691-6) was assigned for all forms of aluminum oxide instead of the CAS No. 1333-84-2 as indicated above. Composition Comments: For the full text of R phrases mentioned in this section, see Section 16. 3. HAZARDS IDENTIFICATION Most Important Hazards: - If crushed or handled extensively, dust may evolve and can be irritating to the eyes, skin, and respiratory tract. Adverse Human Health Effects: - The following medical conditions may be aggravated by exposure to the product: asthma, chronic lung disease, and skin rashes. - If the product contacts the skin with water, it may leave a stain of insoluble products on the skin. -

SDS Simple Cane Alcoholic Distillate
SDS In compliance with HCS/HazCom 2012 SAFETY DATA SHEET Product: Simple Cane Alcoholic Distillate - DASC Revision: 00 Date: 04/22/2021 Pages: 1 /16 1 - IDENTIFICATION Product identifier: Simple Cane Alcoholic Distillate - DASC. Recommended use of the chemical and restrictions Used in the pharmaceutical, cosmetics and beverages industry. on use: Company: Biosev Bioenergia S/A. Rod. Armando de Salles Oliveira, Km 346,3, CEP: 14176-500, Address: Sertãozinho - SP – Brasil. Telephone number: (+55 16) 3946-3900. Emergency telephone 0800 940 9199. number: 2 - HAZARDS IDENTIFICATION Flammable liquids – Category 2. Classification of the Serious eye damage/eye irritation – Category 2A. chemical: Specific target organ toxicity – Single exposure – Category 3. Signal word: DANGER H225 Highly flammable liquid and vapour. Hazard statement(s): H319 Causes serious eye irritation. H335 May cause respiratory irritation. Symbol(s): PREVENTION P210 Keep away from heat, hot surfaces, sparks, open flames, Precautionary statement(s) and other sources. No smoking. P233 Keep container tightly closed. P240 Ground and bond container and receiving equipment. SDS In compliance with HCS/HazCom 2012 SAFETY DATA SHEET Product: Simple Cane Alcoholic Distillate - DASC Revision: 00 Date: 04/22/2021 Pages: 2 /16 P241 Use explosions-proof electrical, ventilating, lighting equipment. P242 Use non-sparking tools. P243 Take action to prevent static discharges. P264 Wash hands thoroughly after handling. P280 Wear protective gloves, protective clothing, eye protection, face protection, hearing protection. RESPONSE P304 + P340 IF INHALED: Remove person to fresh air and keep comfortable for breathing. P303 + P361 + P353 IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water or shower. -

Compound Name
Indicate Type of Compound: Compound Name Write your answer here I = ionic, A= acid, M = molecular manganese (II) bromite I Mn(BrO2)2 manganese (II) phosphite I Mn3(PO3)2 rubidium sulfite I Rb2SO3 hydroselenic acid A H2Se(aq) sodium perbromate I NaBrO4 cobalt (III) chromate I Co2(CrO4)3 antimony (V) nitrite I Sb(NO2)5 chloric acid A HClO3(aq) pentaselenium decabromide M Se5Br10 disulfur decachloride M S2Cl10 nickel (III) nitrate I Ni(NO3)3 copper (II) bromide I CuBr2 nickel (II) hydrogen phosphate I NiHPO4 iron (II) hydrogen sulfate I Fe(HSO4)2 bismuth (V) acetate I Bi(C2H3O2)5 sulfurous acid A H2SO3(aq) sulfuric acid A H2SO4(aq) nickel (II) chloride I NiCl2 tin (IV) phosphate I Sn3(PO4)4 mercury (I) iodate I Hg2(IO3)2 Compound Indicate Type of Compound: Write your answer here Formula I = ionic, A= acid, M = molecular Co(HCO3)2 I (with VOS metal) cobalt (II) hydrogen carbonate Cs2S I cesium sulfide Ca(IO2)2 I calcium iodite Ba2C I barium carbide Mn(CO3)2 I (with VOS metal) manganese (IV) carbonate CuBrO2 I (with VOS metal) copper (I) bromite AgHS I silver hydrogen sulfide C9N10 M nonacarbon decanitride CrI2 I (with VOS metal) chromium (II) iodide Mg(NO3)2 I magnesium nitrate HC2H3O2 (aq) A acetic acid HClO2 (aq) A chlorous acid Be(IO4)2 I beryllium periodate HIO4(aq) A periodic acid BaO I barium oxide Cd(BrO3)2 I cadmium bromate Bi(CN)5 I (with VOS metal) bismuth (V) cyanide AuHS I (with VOS metal) gold (I) hydrogen sulfide AuClO I (with VOS metal) gold (I) hypochlorite Na2CO3 I sodium carbonate Indicate Type of Compound: Compound Name -

(CDR) by CASRN Or Accession Number
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) by CASRN or Accession Number For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed by CAS Registry Number (for non-confidential chemicals) or by TSCA Accession Number (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION NUMBER CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME 100016 Benzenamine, 4-nitro- 10042769 Nitric acid, strontium salt (2:1) 10006287 Silicic acid (H2SiO3), potassium salt (1:2) 10043013 Sulfuric acid, aluminum salt (3:2) 1000824 Urea, N-(hydroxymethyl)- 10043115 Boron nitride (BN) 100107 Benzaldehyde, 4-(dimethylamino)- 10043353 Boric acid (H3BO3) 1001354728 4-Octanol, 3-amino- 10043524 Calcium chloride (CaCl2) 100174 Benzene, 1-methoxy-4-nitro- 100436 Pyridine, 4-ethenyl- 10017568 Ethanol, 2,2',2''-nitrilotris-, phosphate (1:?) 10043842 Phosphinic acid, manganese(2+) salt (2:1) 2,7-Anthracenedisulfonic acid, 9,10-dihydro- 100447 Benzene, (chloromethyl)- 10017591 9,10-dioxo-, sodium salt (1:?) 10045951 Nitric acid, neodymium(3+) salt (3:1) 100185 Benzene, 1,4-bis(1-methylethyl)- 100469 Benzenemethanamine 100209 1,4-Benzenedicarbonyl dichloride 100470 Benzonitrile 100210 1,4-Benzenedicarboxylic acid 100481 4-Pyridinecarbonitrile 10022318 Nitric acid, barium salt (2:1) 10048983 Phosphoric acid, barium salt (1:1) 9-Octadecenoic acid (9Z)-, 2-methylpropyl 10049044 Chlorine oxide (ClO2) 10024472 ester Phosphoric acid, -

A Brief Introduction to Qualitative Analysis for Use in Instruction In
CORNELL UNIVERSITY LIBRARY GIFT OF Professor CaldiArell QD 83.M48T894"'"""'''-'''"^ qualitative miimiiifi'iIII'S™"'''"" '° anal 3 1924 012 380 501 p^ Cornell University Library The original of tliis book is in tlie Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31924012380501 BRIEF INTRODUCTION TO Qualitative Analysis: FOR USE IN INSTRUCTION IN CHEMICAL LABORATORIES. BY LUDWIG MEDICUS, PnOFESSOK OF CHEMISTRY IN THE UNIVERSITY AT WUEZBURG. TRANSLATED FROM THE FOURTH AND FIFTH GERMAN EDITIONS, WITH ADDITIONS, BY JOHN MAESHALL, ASSISTANT PBOFESSOR OF CHEMISTRY IN THE MEDICAL DEPARTMENT OF THE UNIVERSITY OP PENNSYLVANIA. THIRD EDITION. PHILADELPHIA: PRINTED BY J. B. LTPPINCOTT COMPANY. 1894. %t^S\ C s^ Copyright, 1891, by John Mabshali.. TRANSLATOR'S PREFACE, The merit of Medicus' " Qualitative Analysis," and its popularity, which is shown by its having already passed through five editions in the German language, led to this translation. The translator has taken the liberty of rearranging the elements in the first part of the book into groups, to corre- spond with their precipitation by group reagents, and also of adding two tables and amplifying the text to the extent of about forty pages. J. M. Philadelphia, 1892. PREFACE TO THE SECOND EDITION. In the second edition a number of additions and changes have been made in the parts treating of the methods of procedure in the separation of the bases into groups. A table showing the solubility of many of the salts of the commonly occurring metals has also been added. J. -

Potassium Permanganate Safety Data Sheet According to Federal Register / Vol
Potassium Permanganate Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 02/04/2014 Revision date: 02/13/2018 Supersedes: 02/13/2018 Version: 1.1 SECTION 1: Identification 1.1. Identification Product form : Substance Substance name : Potassium Permanganate CAS-No. : 7722-64-7 Product code : LC19850 Formula : KMnO4 Synonyms : permanganate of potash / potassium salt permanganic acid 1.2. Recommended use and restrictions on use Use of the substance/mixture : Oxidant Bleaching agent Reagent Disinfectant Deodorizer Algicide Dyestuff/pigment: component Medicine Laboratory chemical Food industry: additive Insecticide Germicide Recommended use : Laboratory chemicals Restrictions on use : Not for food, drug or household use 1.3. Supplier LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazard(s) identification 2.1. Classification of the substance or mixture GHS-US classification Oxidizing solids Category 2 H272 May intensify fire; oxidizer Acute toxicity (oral) H302 Harmful if swallowed Category 4 Hazardous to the aquatic H400 Very toxic to aquatic life environment - Acute Hazard Category 1 Hazardous to the aquatic H410 Very toxic to aquatic life with long lasting effects environment - Chronic Hazard Category 1 Full text of H statements : see section 16 2.2. GHS Label elements, including precautionary statements GHS-US labeling Hazard pictograms (GHS-US) : GHS03 GHS07 GHS09 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H272 - May intensify fire; oxidizer H302 - Harmful if swallowed 02/13/2018 EN (English US) Page 1 Potassium Permanganate Safety Data Sheet according to Federal Register / Vol. -
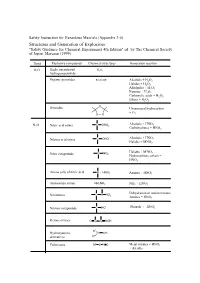
Appendix 3-4 Structures and Generation of Explosives
Safety Instruction for Hazardous Maerials (Appendix 3-4) Structures and Generation of Explosives "Safety Guidance for Chemical Experiments 4th Edition" ed. by The Chemical Society of Japan, Maruzen (1999) Bond Explosive compounds Chemical structures Generation reaction O-O High concentrated H2O2 hydrogen peroxide Organic peroxides R-O-O-R' Alcohols + H2O2 Halides + H2O2 Aldehydes + H2O2 Ketones + H2O2 Carboxylic acids + H2O2 Ethers + H2O2 O Ozonides Unsaturated hydrocarbon + O O O 3 Alcohols + HNO N-O Nitric acid esters C ONO2 3 Carbohydrates + HNO3 Nitrous acid esters C ONO Alcohols + HNO2 Halides + MNO2 Halides + M'NO2 Nitro compounds C NO2 Hydrocarbons, others + HNO3 Amine salts of nitric acid N HNO3 Amines + HNO3 Ammonium nitrate NH4NO3 NH3 + HNO3 Dehydration of amino nitrates Nitramines C N NO2 Amines + HNO3 Nitroso compounds C NO Phenols + HNO2 Ketone oximes O C C NOH R1 Hydroxyamine N OH derivatives R2 Fulminates M' O N C Metal nitrates + HNO3 + Alcohls X-O Dichlorine Heptoxide Cl2O7 Amine salts of perchloric acid N HClO4 Perchloric acid esters C OClO3 Alcohols + HClO4 Perchloryl compounds C ClO3 C H + FClO3 Chloric acid HClO3 M'ClO3 + Acids Chlorine dioxide ClO2 M'ClO2 + Acids Heavy metal salts of KClO + Heavy metal salts, M'ClO 3 chloric acid 3 Hg, Ag, Pb Ammonium chlorate NH ClO M'ClO3 + NH3 or 4 3 Ammonium salts Amine salts of chloric N HClO3 M'ClO + Amine salts acid 3 Chloric acid esters C OClO2 Chlorous acid HClO2 Hypochlorous acid HClO Chlorine monooxide Cl2O Salts of hypochlorous acid M'ClO Hypochlorous acid esters C OCl -

New York City Department of Environmental Protection Community Right-To-Know: List of Hazardous Substances
New York City Department of Environmental Protection Community Right-to-Know: List of Hazardous Substances Updated: 12/2015 Definitions SARA = The federal Superfund Amendments and Reauthorization Act (enacted in 1986). Title III of SARA, known as the Emergency Planning and Community Right-to-Act, sets requirements for hazardous chemicals, improves the public’s access to information on chemical hazards in their community, and establishes reporting responsibilities for facilities that store, use, and/or release hazardous chemicals. RQ = Reportable Quantity. An amount entered in this column indicates the substance may be reportable under §304 of SARA Title III. Amount is in pounds, a "K" represents 1,000 pounds. An asterisk following the Reporting Quantity (i.e. 5000*) will indicate that reporting of releases is not required if the diameter of the pieces of the solid metal released is equal to or exceeds 100 micrometers (0.004 inches). TPQ = Threshold Planning Quantity. An amount entered in this column reads in pounds and indicates the substance is an Extremely Hazardous Substance (EHS), and may require reporting under sections 302, 304 & 312 of SARA Title III. A TPQ with a slash (/) indicates a "split" TPQ. The number to the left of the slash is the substance's TPQ only if the substance is present in the form of a fine powder (particle size less than 100 microns), molten or in solution, or reacts with water (NFPA rating = 2, 3 or 4). The TPQ is 10,000 lb if the substance is present in other forms. A star (*) in the 313 column= The substance is reportable under §313 of SARA Title III. -

The Reaction of Barium Manganate with Acids and Their Precursors
Indian Journal of Chemistry Vol. 38A. September 1999, pp.966-968 The reaction of barium manganate with fraction data were collected on a Phillips PW 3710 acids and their precursors diffractometer, with a Cu monochromator. Synthesis oj barium manganate (VI) Liszlo Kotai, Agnes Keszler, Janos Pato. Sandor Holly Chemical Research Center, Institute of Chemistry. KMn04 (15.8 g) was dissolved in 300 ml of water, Hungarian Academy of Sciences then BaCI2.2Hp (24.9 g dissolved in 100 ml of water), H-1025, Budapest, Pusztaseri u. 59-67, Hungary KOH (56 g dissolved in 100 ml of water) and KI (2.0 g and dissolved in 20 ml of water) were added with vigorous Kalyan K Banerji ' Department of Chemistry, J N V University, stirring. The mixture was boiled for 15 min, cooled, Jodhpur 342 005, India filtered, and washed. The permanganate-free product was dried at 105° C for I h, then the traces of water were Received 30 November 1998; revised 4 Mal' 1999 removed by azeotropic distillation with benzene (yield - 100%). Analysis (found/calc. fo r BaMn0 ): Ba 53.69/ 4 A simple and easy preparative route to obtain permangani c ac id 53.59%; Mn 21.40121.44%. and permanganate salts from barium manganate and sulphuric acid is described. Sulphuric acid reacts with bari um manganate to pro duce sparingly soluble bariulll sulphate and well-soluble permanga Synthesis ojpermanganic acid nic ac id or bariulll permanganate, these in turn can be usee! to pre To barium manganate (2.56 g, 0 .0 I mol) suspended pare ot her metal permanganates. -
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) in Alphabetical Order
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) in Alphabetical Order For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed in alphabetical order by CA Index Name (for non-confidential chemicals) or by generic chemical name (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME NUMBER (Benzoquinolinyl)-(alkylimidazolyl)indenedione 1(3H)-Isobenzofuranone, 6-(dimethylamino)-3,3- deriv., compd. with alkanoic acid 56907 bis[4-( dimethylamino)phenyl]- 1552427 (Substituted-5-sulfophenyl heteromonocycle) azo- 1,10-Decanediamine 646253 (substituted 2-hydroxyphenyl), iron complex, 1,10-Phenanthroline 66717 sodium salt 20736 1,12-Dodecanediol 5675514 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 57501 1,1'-Bicyclohexyl 92513 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 1,1'-Biphenyl 92524 O-.alpha.-D-galactopyranosyl-( 1.fwdarw.6)- 512696 1,1'-Biphenyl, 4,4'-diisocyanato-3,3'-dimethyl- 91974 .alpha.-D-Glucopyranoside, .beta.-D-fructofuranosyl 1,1-Cyclopropanedicarboxylic acid, 1,1-dimethyl O-.alpha.-D-galactopyranosyl-( 1.fwdarw.6)-O- ester 6914712 .alpha.-D-g alactopyranosyl-(1.fwdarw.6)- 470553 1,2,3,4-Butanetetracarboxylic acid, 1,2,3,4- .alpha.-D-Glucopyranoside, .beta.-D- tetrakis(1,2,2,6,6-pentamethyl-4 -piperidinyl) ester 91788839 fructofuranosyl, benzoate 12738646 1,2,3,4-Butanetetracarboxylic acid, 1,2,3,4- .alpha.-D-Glucopyranoside,