Coevolutionary Arms Race Versus Host Defense Chase in a Tropical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Self-Repair and Self-Cleaning of the Lepidopteran Proboscis
Clemson University TigerPrints All Dissertations Dissertations 8-2019 Self-Repair and Self-Cleaning of the Lepidopteran Proboscis Suellen Floyd Pometto Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Recommended Citation Pometto, Suellen Floyd, "Self-Repair and Self-Cleaning of the Lepidopteran Proboscis" (2019). All Dissertations. 2452. https://tigerprints.clemson.edu/all_dissertations/2452 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. SELF-REPAIR AND SELF-CLEANING OF THE LEPIDOPTERAN PROBOSCIS A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy ENTOMOLOGY by Suellen Floyd Pometto August 2019 Accepted by: Dr. Peter H. Adler, Major Advisor and Committee Co-Chair Dr. Eric Benson, Committee Co-Chair Dr. Richard Blob Dr. Patrick Gerard i ABSTRACT The proboscis of butterflies and moths is a key innovation contributing to the high diversity of the order Lepidoptera. In addition to taking nectar from angiosperm sources, many species take up fluids from overripe or sound fruit, plant sap, animal dung, and moist soil. The proboscis is assembled after eclosion of the adult from the pupa by linking together two elongate galeae to form one tube with a single food canal. How do lepidopterans maintain the integrity and function of the proboscis while foraging from various substrates? The research questions included whether lepidopteran species are capable of total self- repair, how widespread the capability of self-repair is within the order, and whether the repaired proboscis is functional. -

Backebergia Militaris (Audot) H. Bravo Hollis Backebergia Militaris (Audot) H
2021/09/24 13:59 1/18 BACKEBERGIA MILITARIS (AUDOT) H. BRAVO HOLLIS BACKEBERGIA MILITARIS (AUDOT) H. BRAVO HOLLIS CACTUS & Co. 2 (8) 2004 Version pdf INTRODUCTION Backebergia militaris (H. Bravo ex Sánchez- Mejorada, 1973) has always been a difficult plant for which to find a taxonomical position. It is included in the tribe Pachycereeae Buxbaum, sub-tribe Pachycere- inae Buxbaum. However, its morphology and life cycle are distinct from those of all the other taxa included. Furthermore, it has a character which is unique in the whole Cactaceae family, that is, it bears an inflores- cence with genetically determinate growth, a decidu- ous one, which for this exclusive character deserves a specific term, that I define as “Tiponche” (pron. teep- ònche), from the vernacular name by which the species is known in its distribution area. The present paper describes for the first time the deciduous character of the inflorescence, and how this adaptation influences the dynamics of the life cycle of B. militaris. Furthermore, its functional significance is investigated, and related to a probable, sophisticated mutual symbiosis between the plant and a lepidopter- an of the Pyraloidea, of a yet undetermined species. This study is the result of bibliographic research and of observations carried out both in the wild, dur- ing a survey in March 1994 in a locality within the dis- tribution area of B. militaris, and with the dissection of authorized 1) samples collected on the same occasion. NOTES ON THE STUDIES CARRIED OUT ON BACKEBERGIA MILITARIS The species was discovered some 150 years ago, but the encounters between B. -
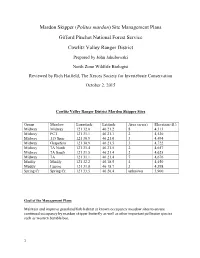
Mardon Skipper Site Management Plans
Mardon Skipper (Polites mardon) Site Management Plans Gifford Pinchot National Forest Service Cowlitz Valley Ranger District Prepared by John Jakubowski North Zone Wildlife Biologist Reviewed by Rich Hatfield, The Xerces Society for Invertebrate Conservation October 2, 2015 Cowlitz Valley Ranger District Mardon Skipper Sites Group Meadow Longitude Latitude Area (acres) Elevation (ft.) Midway Midway 121 32.0 46 21.2 8 4,313 Midway PCT 121 31.1 46 21.1 2 4,530 Midway 115 Spur 121 30.9 46 21.0 3 4,494 Midway Grapefern 121 30.9 46 21.5 3 4,722 Midway 7A North 121 31.4 46 21.5 2 4,657 Midway 7A South 121 31.5 46 21.4 2 4,625 Midway 7A 121 31.1 46 21.4 7 4,676 Muddy Muddy 121 32.2 46.18.5 4 4,450 Muddy Lupine 121 31.8 46 18.7 3 4,398 Spring Cr Spring Cr. 121 33.5 46 20.4 unknown 3,900 Goal of the Management Plans Maintain and improve grassland/forb habitat at known occupancy meadow sites to ensure continued occupancy by mardon skipper butterfly as well as other important pollinator species such as western bumble bee. 1 Introduction On the Gifford Pinchot National Forest (GPNF), mardon skippers were first detected on the Mt. Adams Ranger District (MTA) in 2000 and on Cowlitz Valley Ranger District (CVRD) in 2002. Mardon skippers are known to inhabit ten, upland dry grassy meadows on the CVRD. Portions of the meadows are mesic and are unsuitable mardon skipper habitat. -

197 Section 9 Sunflower (Helianthus
SECTION 9 SUNFLOWER (HELIANTHUS ANNUUS L.) 1. Taxonomy of the Genus Helianthus, Natural Habitat and Origins of the Cultivated Sunflower A. Taxonomy of the genus Helianthus The sunflower belongs to the genus Helianthus in the Composite family (Asterales order), which includes species with very diverse morphologies (herbs, shrubs, lianas, etc.). The genus Helianthus belongs to the Heliantheae tribe. This includes approximately 50 species originating in North and Central America. The basis for the botanical classification of the genus Helianthus was proposed by Heiser et al. (1969) and refined subsequently using new phenological, cladistic and biosystematic methods, (Robinson, 1979; Anashchenko, 1974, 1979; Schilling and Heiser, 1981) or molecular markers (Sossey-Alaoui et al., 1998). This approach splits Helianthus into four sections: Helianthus, Agrestes, Ciliares and Atrorubens. This classification is set out in Table 1.18. Section Helianthus This section comprises 12 species, including H. annuus, the cultivated sunflower. These species, which are diploid (2n = 34), are interfertile and annual in almost all cases. For the majority, the natural distribution is central and western North America. They are generally well adapted to dry or even arid areas and sandy soils. The widespread H. annuus L. species includes (Heiser et al., 1969) plants cultivated for seed or fodder referred to as H. annuus var. macrocarpus (D.C), or cultivated for ornament (H. annuus subsp. annuus), and uncultivated wild and weedy plants (H. annuus subsp. lenticularis, H. annuus subsp. Texanus, etc.). Leaves of these species are usually alternate, ovoid and with a long petiole. Flower heads, or capitula, consist of tubular and ligulate florets, which may be deep purple, red or yellow. -

Two New Records for the Appalachian Grizzled Skipper (Pyrgus Wyandot)
Banisteria, Number 24, 2004 © 2004 by the Virginia Natural History Society Status of the Appalachian Grizzled Skipper (Pyrgus centaureae wyandot) in Virginia Anne C. Chazal, Steven M. Roble, Christopher S. Hobson, and Katharine L. Derge1 Virginia Department of Conservation and Recreation Division of Natural Heritage 217 Governor Street Richmond, Virginia 23219 ABSTRACT The Appalachian grizzled skipper (Pyrgus centaureae wyandot) was documented historically (primarily from shale barren habitats) in 11 counties in Virginia. Between 1992 and 2002, staff of the Virginia Department of Conservation and Recreation, Division of Natural Heritage, conducted 175 surveys for P. c. wyandot at 75 sites in 12 counties. The species was observed at only six sites during these surveys, representing two new county records. All observations since 1992 combined account for <80 individuals. Due to forest succession and threats from gypsy moth control measures, all recent sites for P. c. wyandot in Virginia may be degrading in overall habitat quality. Key words: Lepidoptera, Pyrgus centaureae wyandot, conservation, shale barrens, Virginia. INTRODUCTION wyandot) in Virginia. Parshall (2002) provides a comprehensive review of the nomenclature and The Appalachian grizzled skipper (Pyrgus taxonomy of P. c. wyandot. Most authors classify this centaureae wyandot) has a rather fragmented range, skipper as a subspecies of the Holarctic Pyrgus occurring in northern Michigan as well as portions of centaureae (e.g., Opler & Krizek, 1984; Iftner et al., Ohio, Pennsylvania, Maryland, West Virginia, and 1992; Shuey, 1994; Allen, 1997; Opler, 1998; Virginia; isolated historical records are known from Glassberg, 1999; Parshall, 2002), although some Kentucky, New York, New Jersey, North Carolina, and lepidopterists treat it as a full species (Shapiro, 1974; the District of Columbia (Opler, 1998; NatureServe, Schweitzer, 1989; Gochfeld & Burger, 1997). -

Grizzled Skipper
Species: Grizzled Skipper (Pyrgus wyandot) Global Rank: G1G2Q State Rank: S1 State Wildlife Action Plan: Immediate Concern Responsibility Species Climate Change Vulnerability: Highly Vulnerable Confidence: Very High Note: This assessment is expected to be similar for other butterflies of specialized or moderately specialized forest clearing habitats, with specific food plants, habitats exposed to gypsy moth spray; and lacking a fire resistant dormant stage (larval and/or pupal). Some examples: - Frosted Elfin (Callophrys irus); Global Rank G3, State Rank S2; Caterpillar hostplant Wild Indigo (Baptisia tinctoria); Habitat typically grassy (Andropogon spp.) openings in oak habitats on sandy rocky soils; sometimes found in disturbed areas with hostplant such as powerline right-of-ways. - Persius Duskywing (Erynnis persius); Global Rank G5T1T3, State Rank S1; Caterpillar hostplant Wild Indigo (Baptisia tinctoria); Habitats include pitch pine- scrub oak barrens, scrubby ridgetops, or powerline right-of-ways within such settings with sandy-gravelly soils. - Northern Metalmark (Calephelis borealis), Global Rank G3G4, State Rank S2; Caterpillar hostplant Round-leaved Ragwort (Senecio obovatus); Habitats are openings within forested or wooded areas such as natural outcrops, shale or limestone barrens, glades or powerline right-of-ways. Habitat (adapted from NatureServe 2008 and Schweitzer 1989): The Grizzled Skipper butterfly is an Appalachian Mountain habitat specialist that requires shale barren habitats with abundant exposed crumbly rock or soil. Shale barrens are semi-open shale slopes with sparse herbaceous vegetation and tend to be surrounded by scrubby oak or oak-hickory woodlands, often with a component of Virginia Pine (Pinus virginiana). A natural area which meets the habitat requirements should maintain itself as a shale barren. -

A Revision of the New World Plant-Mining Moths of the Family
Smithsonian Institution Scholarly Press SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 625 A Revision of the New World Plant-Mining Moths of the Family (Lepidoptera: Nepticuloidea) Donald R. Davis and Jonas R. Stonis SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions in History and Technology Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions from the United States National Herbarium Smithsonian Contributions in Visual and Material Culture Smithsonian Contributions to Zoology In these series, the Institution pubHshes small papers and full-scale monographs that report the research and collections of its various museums and bureaus. The Contributions Series are distributed by mailing lists to Ubraries, universities, and similar institutions through- out the world. Manuscripts submitted for series publication are received by the Smith- sonian Institution Scholarly Press from authors with direct affiliation with the various Smithsonian museums or bureaus and are subject to peer review and review for compliance with manuscript preparation guidelines. -

Redalyc.Arctiidae (Insecta: Lepidoptera) Da Estação Biológica
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Gianluppi Ferro, Viviane; Rezende Diniz, Ivone Arctiidae (Insecta: Lepidoptera) da Estação Biológica de Boracéia (Salesópolis, São Paulo, Brasil) Biota Neotropica, vol. 7, núm. 3, septiembre-diciembre, 2007, pp. 331-338 Instituto Virtual da Biodiversidade Campinas, Brasil Disponível em: http://www.redalyc.org/articulo.oa?id=199114292032 Como citar este artigo Número completo Sistema de Informação Científica Mais artigos Rede de Revistas Científicas da América Latina, Caribe , Espanha e Portugal Home da revista no Redalyc Projeto acadêmico sem fins lucrativos desenvolvido no âmbito da iniciativa Acesso Aberto Arctiidae (Insecta: Lepidoptera) da Estação Biológica de Boracéia (Salesópolis, São Paulo, Brasil) Viviane Gianluppi Ferro1,3 & Ivone Rezende Diniz2 Biota Neotropica v7 (n3) – http://www.biotaneotropica.org.br/v7n3/pt/abstract?inventory+bn03107032007 Recebido em 20/06/07 Versão reformulada recebida em 13/09/07 Publicado em 16/10/07 1Programa de Pós-Graduação em Ecologia, Instituto de Ciências Biológicas, Universidade de Brasília – UnB, CP 04457, CEP 70919-970, Brasília, DF, Brasil 2Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade de Brasília – UnB, CEP 70910-900, Brasília, DF, Brasil, e-mail: [email protected] 3Autor para correspondência: Viviane Gianluppi Ferro, e-mail: [email protected] Abstract Ferro, VG. & Diniz, IR. Arctiidae (Insecta: Lepidoptera) of the Boracéia Biological Station (Salesópolis, São Paulo, Brazil). Biota Neotrop. Sep/Dez 2007 vol. 7, no. 3 http://www.biotaneotropica.org.br/v7n3/pt/abstra ct?inventory+bn03107032007. ISSN 1676-0603. A checklist of the Arctiidae moth species with occurrence in the Boracéia Biological Station (EBB) is presented. -

Native Grasses Benefit Butterflies and Moths Diane M
AFNR HORTICULTURAL SCIENCE Native Grasses Benefit Butterflies and Moths Diane M. Narem and Mary H. Meyer more than three plant families (Bernays & NATIVE GRASSES AND LEPIDOPTERA Graham 1988). Native grasses are low maintenance, drought Studies in agricultural and urban landscapes tolerant plants that provide benefits to the have shown that patches with greater landscape, including minimizing soil erosion richness of native species had higher and increasing organic matter. Native grasses richness and abundance of butterflies (Ries also provide food and shelter for numerous et al. 2001; Collinge et al. 2003) and butterfly species of butterfly and moth larvae. These and moth larvae (Burghardt et al. 2008). caterpillars use the grasses in a variety of ways. Some species feed on them by boring into the stem, mining the inside of a leaf, or IMPORTANCE OF LEPIDOPTERA building a shelter using grass leaves and silk. Lepidoptera are an important part of the ecosystem: They are an important food source for rodents, bats, birds (particularly young birds), spiders and other insects They are pollinators of wild ecosystems. Terms: Lepidoptera - Order of insects that includes moths and butterflies Dakota skipper shelter in prairie dropseed plant literature review – a scholarly paper that IMPORTANT OF NATIVE PLANTS summarizes the current knowledge of a particular topic. Native plant species support more native graminoid – herbaceous plant with a grass-like Lepidoptera species as host and food plants morphology, includes grasses, sedges, and rushes than exotic plant species. This is partially due to the host-specificity of many species richness - the number of different species Lepidoptera that have evolved to feed on represented in an ecological community, certain species, genus, or families of plants. -
Investigating Suburban Micromoth Diversity Using DNA Barcoding of Malaise Trap Samples
Urban Ecosyst DOI 10.1007/s11252-016-0597-2 Investigating suburban micromoth diversity using DNA barcoding of malaise trap samples Kaare Aagaard1 & Kai Berggren2 & Paul DN Hebert3 & Jayme Sones3 & Beverly McClenaghan3 & Torbjørn Ekrem1 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Micromoths can be challenging to identify based Introduction on morphology and are frequently omitted in assessments of moth diversity. However, their species richness and biology Urban ecology is a field in constant growth (Cressey 2015). make them important components of terrestrial ecosystems. In Traditionally, birds, mammals and flowering plants have been this study we identified 1227 micromoths from a suburban considered in urban ecology studies, but butterflies and moths garden at 63° north using DNA barcoding of Malaise trap are now often also investigated (Goode 2014). The larger samples. We recorded 78 different species with the 11 most moth species are reasonably well known and easy to identify abundant taxa accounting for 82 % of the catch. The remaining in temperate regions, but micromoths require more taxonomic 67 species were represented by fewer than 14 specimens, but expertise and are therefore rarely considered in urban ecology the number was often sufficient to provide a good idea of studies. However, extensive investigations of urban moth phenology. The larvae of these 78 species all feed on plants communities were recently undertaken in Scotland (Lintott common in suburban environments. We show that when et al. 2014) and Michigan, USA (Rice and White 2015). facilitated by identifications through DNA barcoding, Malaise Urbanization is generally supposed to contribute to biodi- traps provide interesting insights into the micromoth commu- versity loss (McKinney 2002), but only a few large cities in nities of suburban environments that might otherwise be Norway have an extreme urban structure. -

Evaluation of Host Plant Resistance Against Sunflower Moth, <I
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations and Student Research in Entomology Entomology, Department of 8-2017 Evaluation of host plant resistance against sunflower moth, Homoeosoma electellum (Hulst), in cultivated sunflower in western Nebraska Dawn M. Sikora University of Nebraska - Lincoln Follow this and additional works at: http://digitalcommons.unl.edu/entomologydiss Part of the Entomology Commons Sikora, Dawn M., "Evaluation of host plant resistance against sunflower moth, Homoeosoma electellum (Hulst), in cultivated sunflower in western Nebraska" (2017). Dissertations and Student Research in Entomology. 50. http://digitalcommons.unl.edu/entomologydiss/50 This Article is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations and Student Research in Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Evaluation of host plant resistance against sunflower moth, Homoeosoma electellum (Hulst), in cultivated sunflower in western Nebraska by Dawn Sikora A THESIS Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment of Requirements For the Degree of Master of Science Major: Entomology Under the Supervision of Professors Jeff Bradshaw and Gary Brewer Lincoln, Nebraska August, 2017 Evaluation of host plant resistance against sunflower moth, Homoeosoma electellum (Hulst), in cultivated sunflower in western Nebraska Dawn Sikora, M.S. University of Nebraska, 2017 Advisors: Jeff Bradshaw and Gary Brewer Sunflower moth, Homoeosoma electellum, is a serious pest of oilseed and confection sunflowers. In this study, we determine efficacy of resistance against this pest. Thirty commercial hybrids, inbreds, and varieties of sunflowers were tested in replicated field and laboratory studies. -

Moths and Butterflies
LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004MOTHS LJL©2004 LJL©2004AND BUTTERFLIES LJL©2004 LJL©2004 (LEPIDOPTERA) LJL©2004 LJL©2004 LJL©2004 FROM LJL©2004 BAHÍA LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004HONDA LJL©2004 LJL©2004 AND CANALES LJL©2004 LJL©2004 DE TIERRA LJL©2004 ISLANDLJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004(VERAGUAS, LJL©2004 LJL©2004 PANAMA LJL©2004) LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004 LJL©2004