Drug Utilisation of Codeine in Children: EMA Analyses of the Health Improvement Network and of the IMS Health German Databases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metamizol Suicide - Lethal Outcome Despite Maximum Therapy
Toxichem Krimtech 2012;79(2):71 Report from the Clinical Toxicology Committee of the Society of Toxicological and Forensic Chemistry (GTFCh) Metamizol Suicide - Lethal Outcome Despite Maximum Therapy Detlef Haase, Sabine Hübner, Silke Kunellis, Gerlinde Kotzerke, Harald König Helios Hospital Schwerin, Institute for Laboratory and Transfusion Medicine, Toxicology Department, Wismarsche Straße 393-397, D-19049 Schwerin, Germany Abstract A 70 year old female patient, suffering for years from rheumatoid arthritis and associated chronic pain was referred to the hospital by an emergency physician. Her blood pressure was no longer measurable; a hemiparesis has developed. A preliminary examination was carried out in the emergency department by a neurologist and a cerebral CT was requested. Immediately after examination, the patient suffered from hypodynamic cardiac arrest and had to be cardiopulmonary resuscitated. After stabilisation she was transferred to the Stroke Unit, where tonic-clonic convulsive seizures occurred. Toxicological general-unknown analysis of the patient's serum confirmed a suspected metamizol intoxication. Despite a maximum permissible dose of noradrenaline, she died four days after hospitalisation due to multiple organ failure. 1. Introduction 1.1. Metamizol Metamizol (novaminsulfone), closely related to phenazone and propyphenazone, is the most powerful analgesic and antipyretic of the pyrazolone derivatives and still on the market. 4-N- methyl-aminoantipyrine (MAA) is also effective, but formed through metamizol hydrolysis in the body. Patients with glucose-6-dehydrogenase deficiency should never use metamizol, be- cause a haemolytic crisis could be triggered. In addition, metamizol has a considerable poten- tial for side-effects, of which agranulocytosis is the most significant [1]. Therefore, metamizol is no more licensed in many countries. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Specifications of Approved Drug Compound Library
Annexure-I : Specifications of Approved drug compound library The compounds should be structurally diverse, medicinally active, and cell permeable Compounds should have rich documentation with structure, Target, Activity and IC50 should be known Compounds which are supplied should have been validated by NMR and HPLC to ensure high purity Each compound should be supplied as 10mM solution in DMSO and at least 100µl of each compound should be supplied. Compounds should be supplied in screw capped vial arranged as 96 well plate format. -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
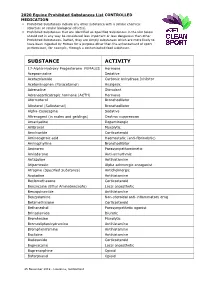
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

National OTC Medicines List
National OTC Medicines List ‐ DraŌ 01 DRAFT National OTC Medicines List Draft 01 Ministry of Public Health of Lebanon This list was prepared under the guidance of His Excellency Minister Waêl Abou Faour andDRAFT the supervision of the Director General Dr. Walid Ammar. Editors Rita KARAM, Pharm D. PhD. Myriam WATFA, Pharm D Ghassan HAMADEH, MD.CPE FOREWORD According to the French National Agency for Medicines and Health Products Safety (ANSM), Over-the-counter (OTC) drugs are medicines that are accessible to patients in pharmacies, based on criteria set to safeguard patients’ safety. Due to their therapeutic class, these medicines could be dispensed without physician’s intervention for diagnostic, treatment initiation or maintenance purposes. Moreover, their dosage, treatment period and Package Insert Leaflet should be suitable for OTC classification. The packaging size should be in accordance with the dosage and treatment period. According to ArticleDRAFT 43 of the Law No.367 issued in 1994 related to the pharmacy practice, and the amendment of Articles 46 and 47 by Law No.91 issued in 2010, pharmacists do not have the right to dispense any medicine that is not requested by a unified prescription, unless the medicine is mentioned in a list which is established by pharmacists and physicians’ syndicates. In this regard, the Ministry of Public Health (MoPH) developed the National OTC Medicines List, and presentedit in a scientific, objective, reliable, and accessible listing. The OTC List was developed by a team of pharmacists and physicians from the Ministry of Public Health (MoPH). In order to ensure a safe and effective self- medicationat the pharmacy level, several pharmaceutical categories (e.g. -

Mucoactive Agents for Airway Mucus Hypersecretory Diseases
Mucoactive Agents for Airway Mucus Hypersecretory Diseases Duncan F Rogers PhD FIBiol Introduction Sputum Profile of Airway Inflammation and Mucus Hypersecretory Phenotype in Asthma, COPD, and CF Which Aspect of Airway Mucus Hypersecretion to Target? Theoretical Requirements for Effective Therapy of Airway Mucus Hypersecretion Current Recommendations for Clinical Use of Mucolytic Drugs Mucoactive Drugs N-Acetylcysteine: How Does it Work? Does it Work? Dornase Alfa Hypertonic Saline Surfactant Analysis Summary Airway mucus hypersecretion is a feature of a number of severe respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). However, each disease has a different airway inflammatory response, with consequent, and presumably linked, mucus hypersecretory phenotype. Thus, it is possible that optimal treatment of the mucus hyper- secretory element of each disease should be disease-specific. Nevertheless, mucoactive drugs are a longstanding and popular therapeutic option, and numerous compounds (eg, N-acetylcysteine, erdosteine, and ambroxol) are available for clinical use worldwide. However, rational recommen- dation of these drugs in guidelines for management of asthma, COPD, or CF has been hampered by lack of information from well-designed clinical trials. In addition, the mechanism of action of most of these drugs is unknown. Consequently, although it is possible to categorize them according to putative mechanisms of action, as expectorants (aid and/or induce cough), mucolytics (thin -

Different Techniques for Analysis of Aspirin, Caffeine, Diclofenac Sodium and Paracetamol: Review Article
ISSN 2692-4374 Pharmaceutical Sciences | Review Article Different techniques for Analysis of Aspirin, Caffeine, Diclofenac Sodium and Paracetamol: Review Article Mahmoud M. Sebaiy1*, Sobhy M. El-Adl1, and Amr A. Mattar1&2 1 Medicinal Chemistry Department, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt. 2 Pharmaceutical Medicinal Chemistry Department, Faculty of Pharmacy, Egyptian Russian University, Badr City, Cairo 11829, Egypt. *Аuthоrcоrrеspоndеncе: Е-mаil: mmsеbаiу@zu.еdu.еg; sеbаiуm@gmаil.cоm.Tеl: 01062780060. Fаx: 0552303266 Submitted: 27 April 2020 Approved: 11 May 2020 Published: 14 May 2020 How to cite this article: Sebaiy MM, El-Adl SM, Mattar AA. Different techniques for Analysis of Aspirin, Caffeine, Diclofenac Sodium and Paracetamol: Review Article. G Med Sci. 2020; 1(1): 013-031. https://www.doi.org/10.46766/thegms.pharma.20042701 Copyright: © 2020 Mahmoud MS. This is an open access article distributed under the Creative Commons Attribution License, which permits unre- stricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTracT Early treatment of pain is of a great importance as unrelieved pain can have profound psychological effects on the patient, and acute pain that is poorly managed initially can degenerate into chronic pain, which may prove to be much more difficult to treat. It is important to assess and treat the article,mental weand will emotional shed the aspects light on of different the pain waysas well of assome its physicalanalgesic aspects. drugs monitoring Although drug and therapyanalysis isusing a mainstay different of techniques pain treatment, in addition physical to methodsthe most such as physiotherapy (including massage and the application of heat and cold), surgery, and drug monitoring are also very valuable. -

Bromhexine Hydrochloride
Bromhexine Hydrochloride sc-204656 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Bromhexine Hydrochloride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Expectorant. Reported to change the structure of bronchial secretions and to increase the volume and reduce the viscosity of sputum. Administered in bronchitis and other respiratory conditions. Normally given by mouth. Synthetic derivative of vasicine, the active principal of Adhatoda vasica. SYNONYMS C14-H20-Br2-N2.HCl, "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", monohydrochloride, "benzylamine, 2-amino-N- cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "benzylamine, 2-amino-N-cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "bromhexin hydrochloride", "N-cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", "N- cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", Aletor, Bisolvomycin, Bisolvon, "Bisolvon Hydrochloride", Bromessina, Bromcilate, Bronkocin, "Dakroy Biciron", Ophtosol, "quaternary ammonium expectorant" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed. -

List Over Prohibited Substances and Withdrawal Times, Valid from January 1St, 2016 A. LIST of PROHIBITED SUBSTANCES
Reviewed 01.06.16 List over prohibited substances and withdrawal times, valid from January 1st, 2016 This list has been developed in collaboration with the other Nordic countries through NEMAC (Nordic Equine Medication and Anti-doping Committee) and is reviewed yearly by the board of DNT (The Norwegian Trotting Association) (in accordance with DNT’s Doping Regulations 2016 § 3, 1st subsection). The List of prohibited substances and withdrawal times consists of the A-list, listing substances and treatment methods that are absolutely prohibited for horses, and the B-list, listing substances that are prohibited in competition; the withdrawal times for these substances as well as treatment methods with withdrawal times. The list may be reviewed several times per year. This list is valid starting from January 1st, 2016 and is enforced until a new list takes effect. A valid list of withdrawal times can be found at any time on DNT’s official website, www.travsport.no. Withdrawal times given on STC’s official website, www.travsport.se, are also valid in Norway. Health certificate and keeping of medical records The trainer is responsible for ensuring that any treatment that requires a withdrawal time is listed in the horse’s health certificate. The start and end dates of the treatment, the name of the treatment/medication/active substance, amount given, method of administration, withdrawal time as well as the name of the veterinarian or other person responsible for the treatment must all be listed in the health certificate in accordance with DNT’s Doping Regulations 2016 § 4. Passport and health certificate should be brought with the horse at all times. -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX