Bromhexine Hydrochloride: Potential Approach to Prevent Or Treat Early Stage COVID-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
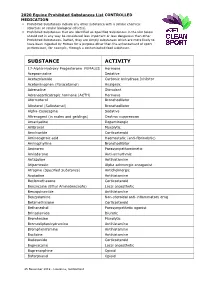
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

National OTC Medicines List
National OTC Medicines List ‐ DraŌ 01 DRAFT National OTC Medicines List Draft 01 Ministry of Public Health of Lebanon This list was prepared under the guidance of His Excellency Minister Waêl Abou Faour andDRAFT the supervision of the Director General Dr. Walid Ammar. Editors Rita KARAM, Pharm D. PhD. Myriam WATFA, Pharm D Ghassan HAMADEH, MD.CPE FOREWORD According to the French National Agency for Medicines and Health Products Safety (ANSM), Over-the-counter (OTC) drugs are medicines that are accessible to patients in pharmacies, based on criteria set to safeguard patients’ safety. Due to their therapeutic class, these medicines could be dispensed without physician’s intervention for diagnostic, treatment initiation or maintenance purposes. Moreover, their dosage, treatment period and Package Insert Leaflet should be suitable for OTC classification. The packaging size should be in accordance with the dosage and treatment period. According to ArticleDRAFT 43 of the Law No.367 issued in 1994 related to the pharmacy practice, and the amendment of Articles 46 and 47 by Law No.91 issued in 2010, pharmacists do not have the right to dispense any medicine that is not requested by a unified prescription, unless the medicine is mentioned in a list which is established by pharmacists and physicians’ syndicates. In this regard, the Ministry of Public Health (MoPH) developed the National OTC Medicines List, and presentedit in a scientific, objective, reliable, and accessible listing. The OTC List was developed by a team of pharmacists and physicians from the Ministry of Public Health (MoPH). In order to ensure a safe and effective self- medicationat the pharmacy level, several pharmaceutical categories (e.g. -

Mucoactive Agents for Airway Mucus Hypersecretory Diseases
Mucoactive Agents for Airway Mucus Hypersecretory Diseases Duncan F Rogers PhD FIBiol Introduction Sputum Profile of Airway Inflammation and Mucus Hypersecretory Phenotype in Asthma, COPD, and CF Which Aspect of Airway Mucus Hypersecretion to Target? Theoretical Requirements for Effective Therapy of Airway Mucus Hypersecretion Current Recommendations for Clinical Use of Mucolytic Drugs Mucoactive Drugs N-Acetylcysteine: How Does it Work? Does it Work? Dornase Alfa Hypertonic Saline Surfactant Analysis Summary Airway mucus hypersecretion is a feature of a number of severe respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). However, each disease has a different airway inflammatory response, with consequent, and presumably linked, mucus hypersecretory phenotype. Thus, it is possible that optimal treatment of the mucus hyper- secretory element of each disease should be disease-specific. Nevertheless, mucoactive drugs are a longstanding and popular therapeutic option, and numerous compounds (eg, N-acetylcysteine, erdosteine, and ambroxol) are available for clinical use worldwide. However, rational recommen- dation of these drugs in guidelines for management of asthma, COPD, or CF has been hampered by lack of information from well-designed clinical trials. In addition, the mechanism of action of most of these drugs is unknown. Consequently, although it is possible to categorize them according to putative mechanisms of action, as expectorants (aid and/or induce cough), mucolytics (thin -

Bromhexine Hydrochloride
Bromhexine Hydrochloride sc-204656 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Bromhexine Hydrochloride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Expectorant. Reported to change the structure of bronchial secretions and to increase the volume and reduce the viscosity of sputum. Administered in bronchitis and other respiratory conditions. Normally given by mouth. Synthetic derivative of vasicine, the active principal of Adhatoda vasica. SYNONYMS C14-H20-Br2-N2.HCl, "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", monohydrochloride, "benzylamine, 2-amino-N- cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "benzylamine, 2-amino-N-cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "bromhexin hydrochloride", "N-cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", "N- cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", Aletor, Bisolvomycin, Bisolvon, "Bisolvon Hydrochloride", Bromessina, Bromcilate, Bronkocin, "Dakroy Biciron", Ophtosol, "quaternary ammonium expectorant" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed. -

List Over Prohibited Substances and Withdrawal Times, Valid from January 1St, 2016 A. LIST of PROHIBITED SUBSTANCES
Reviewed 01.06.16 List over prohibited substances and withdrawal times, valid from January 1st, 2016 This list has been developed in collaboration with the other Nordic countries through NEMAC (Nordic Equine Medication and Anti-doping Committee) and is reviewed yearly by the board of DNT (The Norwegian Trotting Association) (in accordance with DNT’s Doping Regulations 2016 § 3, 1st subsection). The List of prohibited substances and withdrawal times consists of the A-list, listing substances and treatment methods that are absolutely prohibited for horses, and the B-list, listing substances that are prohibited in competition; the withdrawal times for these substances as well as treatment methods with withdrawal times. The list may be reviewed several times per year. This list is valid starting from January 1st, 2016 and is enforced until a new list takes effect. A valid list of withdrawal times can be found at any time on DNT’s official website, www.travsport.no. Withdrawal times given on STC’s official website, www.travsport.se, are also valid in Norway. Health certificate and keeping of medical records The trainer is responsible for ensuring that any treatment that requires a withdrawal time is listed in the horse’s health certificate. The start and end dates of the treatment, the name of the treatment/medication/active substance, amount given, method of administration, withdrawal time as well as the name of the veterinarian or other person responsible for the treatment must all be listed in the health certificate in accordance with DNT’s Doping Regulations 2016 § 4. Passport and health certificate should be brought with the horse at all times. -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2019) Revision 4 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2019) Revision 4 Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Overview of Planned Or Ongoing Studies of Drugs for the Treatment of COVID-19
Version of 16.06.2020 Overview of planned or ongoing studies of drugs for the treatment of COVID-19 Table of contents Antiviral drugs ............................................................................................................................................................. 4 Remdesivir ......................................................................................................................................................... 4 Lopinavir + Ritonavir (Kaletra) ........................................................................................................................... 7 Favipiravir (Avigan) .......................................................................................................................................... 14 Darunavir + cobicistat or ritonavir ................................................................................................................... 18 Umifenovir (Arbidol) ........................................................................................................................................ 19 Other antiviral drugs ........................................................................................................................................ 20 Antineoplastic and immunomodulating agents ....................................................................................................... 24 Convalescent Plasma ........................................................................................................................................... -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Current Pharma Research ISSN-2230-7842 CODEN-CPRUE6
Curr. Pharm. Res. 2016, 6(3), 1860-1876 Current Pharma Research ISSN-2230-7842 CODEN-CPRUE6 www.jcpronline.in/ Research Article A novel stability indicating RP-HPLC method development and validation for estimation of Phenylephrine hydrochloride and Bromhexine hydrochloride in their tablet dosage form. Vanita D. Rohit*1, Jinal Tandel1, Payal Chauhan1, Samir Shah2 *1Department of Quality Assurance, Sardar Patel College of Pharmacy, Bakrol, Anand, India, 2Department of Pharmacology, Sardar Patel College of Pharmacy, Bakrol, Anand, India. Received May 21, 2016; received in revised form June 20, 2016; accepted June 20, 2016 *Corresponding author E-mail address: [email protected] ABSTRACT A novel, specific, sensitive, accurate and economical stability indicating RP-HPLC method was developed for the estimation of Phenylephrine hydrochloride and Bromhexine hydrochloride in their tablet dosage form. Separation was achieved on C18 column (250 × 4.6 mm, 5µm) using Optimized Mobile Phase Water: Acetonitrile (60:40 % v/v) (pH adjusted to 4.5 with 1% OPA) and flow rate maintained at 1.0 ml/min was used. Wavelength was monitored at 215 nm. Both the drugs were subjected to acid, base, oxidation, thermal and photolytic degradation conditions. The retention time of PHN and BHX were found to be 3.24 min and 7.11 min respectively. The linearity response was observed in range of 5-25 µg/ml and 4-20 µg/ml of PHN and BHX respectively. Force degradation study revealed that maximum degradation of PHN and BHX was occur in Photolytic degradation. (Standard 19.88%, Sample 18.07% and standard 19.81%, Sample 18.26%) respectively. -

Bisolvon Chesty Forte Oral Contains Bromhexine Hydrochloride 8 Mg
NEW ZEALAND DATA SHEET 1 BISOLVON CHESTY FORTE Bromhexine hydrochloride tablets Bromhexine hydrochloride Oral Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet of Bisolvon Chesty Forte contains bromhexine hydrochloride 8 mg. Each 5mL of Bisolvon Chesty Forte Oral contains bromhexine hydrochloride 8 mg. 3 PHARMACEUTICAL FORM Oral tablet Oral solution 4 CLINICAL PARTICULARS 4.1 THERAPEUTIC INDICATIONS For use as a mucolytic to break down mucus and help clear the chest in conditions accompanied by excessive mucus secretions, such as in the common cold, influenza, infections of the respiratory tract or in other conditions where excess mucus is produced. 4.2 DOSE AND METHOD OF ADMINISTRATION Dose and Method of administration Do not use in children under 6 years of age. Use in children aged 6 to 11 years only on the advice of a doctor, pharmacist or nurse practitioner. bisolchstyfrteliqtab-ccds1-ds3-17aug21 Page 1 Bisolvon Chesty Forte Tablets: Adults and children 12 years and over: One tablet (8 mg) three times a day when necessary. May be increased to two tablets (16 mg) three times a day for the first seven days. Children 6 – 11 years: 1 tablet three times a day when necessary. Bisolvon Chesty Forte Oral Solution: Adults and children 12 years and over: 5 ml (8 mg) three times a day when necessary. May be increased to 10 ml (16 mg) three times a day for the first seven days. Children 6 – 11 years: 5mL (8 mg) three times a day when necessary. The measuring cup provided should be used for dosing. The oral liquid is alcohol-free and sugar-free and therefore suitable for diabetics. -

Antibiotics, Expectorants, and Cough Suppressants
Antibiotics, Expectorants, and Cough Suppressants Center For Cardiac Fitness Pulmonary Rehab The Miriam Hospital Objectives • Review the mechanism of action (MOA), dosing, benefits, and various options for: – Expectorants – Cough suppressants – Antibiotics for moderate to severe COPD exacerbations • Macrolides • Respiratory fluoroquinolones • Pseudomonas aeruginaosa coverage expectorants • Limited data to suggest improvements to lung function or overall feelings of well-being • MOA: – Wakes up the nerve in the stomach and causes an increase in airway secretions • Ex: Guaifenesin, ipecac, bromhexine, ammonium salts – Emetics • Other MOAs: – Decrease in mucous thickness or an enhancement of the mucociliary escalator – Mucociliary escalator • 2 parts: – Mucous-producing goblet cells – Ciliated epithelium • Bacteria become trapped in the mucous • Cilia, which are constantly beating, push bacteria up and out of the throat Expectorants • Guaifenesin – Dose • Extended release tabs – 600 to 1200 mg every 12 hours • Immediate release tabs/syrup – 200 to 400 mg every 4 to 6 hours • Max = 2400 mg/24 hours – MOA • Increases hydration of mucous and facilitates clearance of mucous from cilia – Side effects • Dizziness, drowsiness, headache, nausea/vomiting, stomach upset, hypouricemia, skin rash • Water – Helps thin and clear mucous Cough Suppressants • Centrally-acting MOA: – Suppress cough via an action on the central cough center • Ex: Dextromethorphan, codeine, long-acting morphine, gabapentin (off-label) • Peripherally-acting MOA: – Depending upon -

Review of Cough and Cold Medicines in Children
Review of cough and cold medicines in children 21 April 2009 Review of cough and cold medicines in children Contents 1. Terms of Reference ....................................................................................................3 2. Conflict of interest and disclaimer............................................................................4 3. Setting the scene........................................................................................................4 4. Current product availability and label claims ..........................................................5 5. ‘Coughs and colds’ – what are we treating? ............................................................7 6. Who uses these medicines? What do they expect from them? .............................8 7. Sedation: side-effect or desired effect? ...................................................................9 8. The need for better drugs ........................................................................................10 9. Epidemiology of childhood poisoning....................................................................10 10. Cough and cold medicines: Australia compared with US.................................12 11. TGA, cough and cold medicines and the general public...................................13 12. Dosage considerations.........................................................................................13 13. Review of the evidence.........................................................................................14 13.1 Antitussives