Antibiotics, Expectorants, and Cough Suppressants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Appendix a Common Abbreviations Used in Medication
UNIVERSITY OF AMSTERDAM MASTERS THESIS Impact of Medication Grouping on Fall Risk Prediction in Elders: A Retrospective Analysis of MIMIC-III Critical Care Database Student: SRP Mentor: Noman Dormosh Dr. Martijn C. Schut Student No. 11412682 – SRP Tutor: Prof. dr. Ameen Abu-Hanna SRP Address: Amsterdam University Medical Center - Location AMC Department Medical Informatics Meibergdreef 9, 1105 AZ Amsterdam Practice teaching period: November 2018 - June 2019 A thesis submitted in fulfillment of the requirements for the degree of Master of Medical Informatics iii Abstract Background: Falls are the leading cause of injury in elderly patients. Risk factors for falls in- cluding among others history of falls, old age, and female gender. Research studies have also linked certain medications with an increased risk of fall in what is called fall-risk-increasing drugs (FRIDs), such as psychotropics and cardiovascular drugs. However, there is a lack of consistency in the definitions of FRIDs between the studies and many studies did not use any systematic classification for medications. Objective: The aim of this study was to investigate the effect of grouping medications at different levels of granularity of a medication classification system on the performance of fall risk prediction models. Methods: This is a retrospective analysis of the MIMIC-III cohort database. We created seven prediction models including demographic, comorbidity and medication variables. Medica- tions were grouped using the anatomical therapeutic chemical classification system (ATC) starting from the most specific scope of medications and moving up to the more generic groups: one model used individual medications (ATC level 5), four models used medication grouping at levels one, two, three and four of the ATC and one model did not include med- ications. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Injectable Anesthesia in South American Camelids
INJECTABLE ANESTHESIA IN SOUTH AMERICAN CAMELIDS Thomas Riebold DVM, Diplomate ACVAA Veterinary Teaching Hospital College of Veterinary Medicine Oregon State University Corvallis, Oregon 97331 INTRODUCTION Interest in llamas, and more recently in alpacas, as pets and as breeding and pack animals has led to increased demand for veterinary services for them. While they have some unique species characteristics regarding anesthesia, many of the principles and techniques used in food animal and equine anesthesia also apply to South American camelids. Except for differences in size, anesthetic management of alpacas and llamas is similar. Much like there are species differences between cattle, sheep, and goats in their response to xylazine, it does appear that alpacas require higher doses of sedatives, approximately 10-20%, to obtain the same response that lower doses of sedatives would obtain in llamas. PREANESTHETIC CONSIDERATIONS Consideration for preanesthetic preparation includes fasting, assessment of hematologic and blood chemistry values, venous catheterization, and estimation of bodyweight. The camelid has a stomach divided into three compartments. Therefore, potential complications similar to those of domestic ruminants, regurgitation and aspiration pneumonia, exist during anesthesia. Abdominal tympany as it occurs in anesthetized domestic ruminants does not appear to occur in anesthetized camelids. It is recommended that the animals be fasted 12-18 hours and deprived of water for 8-12 hours. In nonelective cases, this is often not possible and precautions should be taken to avoid aspiration of gastric fluid and ingesta. Fasting neonatal camelids is not advisable because hypoglycemia may result. As in other species, hematologic and blood chemistry values are determined before anesthesia. -

Medicare Part D Excluded Drug List
MEDICARE PART D EXCLUDED DRUGS LIST 2016_updated July 2016 Reason: LIST = multiple reasons it's excluded; "not covered under Part D law" Reason: Not properly listed with FDA = CMS considers it best practice for Part D sponsors to consider the proper listing of a drug product with the FDA as a prerequisite for making a Part D drug coverage determination. The FDA is unable to provide regulatory status determinations through their regular processes if a drug product is not properly listed. Therefore, Part D sponsors should begin the drug coverage determination process by confirming that a prescription drug product national drug code (NDC) is properly listed with the FDA. Reason: DESI = Less Than Effective (LTE) drug for ALL indications Label Name Reason 1ST BASE CRE Bulk Ingredient 2-FUCCOSYLLA PAK LACTO-N Medical Food ABANEU-SL SUB Vitamin/Mineral ABLAVAR INJ 244MG/ML Diagnostic Agent ACACIA EXTRA SOL 1:20 Non-standardized allergenic ACCUCAINE INJ 1% LIST ACD FORMULA SOL A Blood Component ACD-A SOL Blood Component ACLARO PD EMU 4% Cosmetic ACREMONIUM SOL 20000PNU Non-standardized allergenic ACTCT FLEX 3 PAD 4"X4" Not properly listed with FDA ACTHREL INJ 100MCG Diagnostic Agent ACTI ANTIMIC PAD 2"X2" Not properly listed with FDA ACTI ANTIMIC PAD 4"X4" Not properly listed with FDA ACTICOAT 7 PAD 2"X2" Not properly listed with FDA ACTICOAT 7 PAD 4"X5" Not properly listed with FDA ACTICOAT ABS PAD 4"X5" Not properly listed with FDA ACTICOAT MOI PAD 2"X2" Surgical Supply/Medical ACTICOAT MOI PAD 4"X4" Surgical Supply/Medical ACTICOAT MOI PAD -
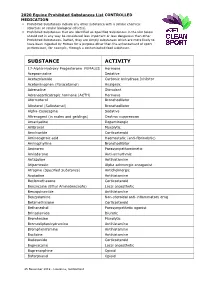
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

“Opioid” Definition Drug Inclusion and Exclusion List
WSHA WSMA Opioid Prescribing Report Medication Inclusion/Exclusion list For purposes of developing WSHA WSMA Opioid Prescribing Report metrics, the following medications tracked in the Prescription Monitoring Program database were included and counted as “opioid prescriptions”: Drug Name ACETAMINOPHEN-CODEINE ASCOMP WITH CODEINE BELLADONNA-OPIUM BUTALB-ACETAMINOPH-CAFF-CODEIN BUTALB-CAFF-ACETAMINOPH-CODEIN BUTALBITAL COMPOUND-CODEINE BUTORPHANOL TARTRATE CARISOPRODOL-ASPIRIN-CODEINE CHERATUSSIN AC CHERATUSSIN DAC CODEINE SULFATE CODEINE-GUAIFENESIN DEMEROL DILAUDID DOLOPHINE HCL DURAMORPH ENDOCET FENTANYL CITRATE FENTORA FIORICET WITH CODEINE FIORINAL WITH CODEINE #3 GUAIATUSSIN AC GUAIFENESIN AC GUAIFENESIN DAC GUAIFENESIN W/CODEINE GUAIFENESIN-CODEINE HYDROCODONE BITARTRATE HYDROCODONE BT-HOMATROPINE MBR HYDROCODONE-ACETAMINOPHEN HYDROCODONE-CHLORPHENIRAMNE ER HYDROCODONE-HOMATROPINE MBR HYDROCODONE-IBUPROFEN HYDROMET HYDROMORPHONE HCL INFUMORPH IOPHEN-C NR LAZANDA LEVORPHANOL TARTRATE LORTAB MEPERIDINE HCL MORPHINE SULFATE NORCO NUCYNTA OPANA OPIUM TINCTURE OXAYDO OXYCODONE HCL OXYCODONE HCL-ASPIRIN OXYCODONE HCL-IBUPROFEN OXYCODONE HYDROCHLORIDE OXYCODONE-ACETAMINOPHEN OXYMORPHONE HCL PENTAZOCINE-NALOXONE HCL PERCOCET PRIMLEV PROMETHAZINE VC-CODEINE PROMETHAZINE-CODEINE PROMETHAZINE-PHENYLEPH-CODEINE ROXICODONE SUBSYS SUFENTANIL CITRATE TRAMADOL HCL TRAMADOL HCL-ACETAMINOPHEN TUSSIGON TUSSIONEX TYLENOL-CODEINE NO.3 TYLENOL-CODEINE NO.4 ULTRACET ULTRAM VICODIN VICODIN ES VICODIN HP VIRTUSSIN AC Other medications, used primarily to -

National OTC Medicines List
National OTC Medicines List ‐ DraŌ 01 DRAFT National OTC Medicines List Draft 01 Ministry of Public Health of Lebanon This list was prepared under the guidance of His Excellency Minister Waêl Abou Faour andDRAFT the supervision of the Director General Dr. Walid Ammar. Editors Rita KARAM, Pharm D. PhD. Myriam WATFA, Pharm D Ghassan HAMADEH, MD.CPE FOREWORD According to the French National Agency for Medicines and Health Products Safety (ANSM), Over-the-counter (OTC) drugs are medicines that are accessible to patients in pharmacies, based on criteria set to safeguard patients’ safety. Due to their therapeutic class, these medicines could be dispensed without physician’s intervention for diagnostic, treatment initiation or maintenance purposes. Moreover, their dosage, treatment period and Package Insert Leaflet should be suitable for OTC classification. The packaging size should be in accordance with the dosage and treatment period. According to ArticleDRAFT 43 of the Law No.367 issued in 1994 related to the pharmacy practice, and the amendment of Articles 46 and 47 by Law No.91 issued in 2010, pharmacists do not have the right to dispense any medicine that is not requested by a unified prescription, unless the medicine is mentioned in a list which is established by pharmacists and physicians’ syndicates. In this regard, the Ministry of Public Health (MoPH) developed the National OTC Medicines List, and presentedit in a scientific, objective, reliable, and accessible listing. The OTC List was developed by a team of pharmacists and physicians from the Ministry of Public Health (MoPH). In order to ensure a safe and effective self- medicationat the pharmacy level, several pharmaceutical categories (e.g. -

Blue Cross and Blue Shield January 2018 5 Tier Basic Drug List
January 2018 5 Tier Basic Drug List Please consider talking to your doctor about prescribing preferred medications, which may help reduce your out-of-pocket costs. This list may help guide you and your doctor in selecting an appropriate medication for you. The drug list is regularly updated. You can view the most up-to-date list, or the specialty drug list, at MyPrime.com. Contents Therapeutic Class Drug List Introduction ....................................................... I Anti-Infective Agents ........................................ 1 How drugs are selected .................................... I Cancer Drugs ................................................... 3 How member payment is determined ............... I Hormones, Diabetes and Related Drugs ......... 5 How to use this list ............................................ I Heart and Circulatory Drugs ............................ 9 Generic drugs .................................................. II Respiratory Agents ........................................ 14 Coverage considerations ................................ III Gastrointestinal Drugs ................................... 16 Specialty drugs .............................................. IV Genitourinary Drugs ....................................... 17 Central Nervous System Drugs ..................... 1 Abbreviation/acronym key .............................. V 8 Pain Relief Drugs ........................................... 21 Neuromuscular Drugs .................................... 23 Supplements ................................................. -

Inhibition of Cough-Reflex Sensitivity by Benzonatate and Guaifenesin In
Respiratory Medicine (2009) 103, 902e906 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough Peter V. Dicpinigaitis a,*, Yvonne E. Gayle a, Gail Solomon b, Richard D. Gilbert c a Albert Einstein College of Medicine and Montefiore Medical Center, 1825 Eastchester Road, Bronx, NY, 10461, USA b Reckitt Benckiser, Inc., 399 Interpace Parkway, Parsippany, NJ, 07054, USA c TKL Research, Inc., 365 West Passaic Street, Rochelle Park, NJ, 07662, USA Received 8 September 2008; accepted 9 December 2008 Available online 1 January 2009 KEYWORDS Summary Cough; Acute cough due to viral upper respiratory tract infection (URI) is the most common form of Antitussive; cough and accounts for tremendous expenditure on prescription and non-prescription cough Guaifenesin; products worldwide. However, few agents have been shown in properly conducted clinical Benzonatate; trials to be effective for cough due to URI. The present study evaluated the effect of benzo- Capsaicin; natate 200 mg (B), guaifenesin 600 mg (G), their combination (B þ G), and placebo (P) on Common cold capsaicin-induced cough in 30 adult nonsmokers with acute URI. On 3 separate days within a 7-day period, 1 h after ingesting randomly assigned study drug in a double-blind fashion, subjects underwent capsaicin cough challenge testing, which involved inhalation of incre- mental doubling concentrations of capsaicin until the concentration of capsaicin inducing 5 or more coughs (C5) was attained. Each subject received 3 of 4 possible study drugs. G (p Z 0.01) but not B (p Z NS) inhibited cough-reflex sensitivity (log C5)relativetoP.The combination of B þ G suppressed capsaicin-induced cough to a greater degree than B alone (p < 0.001) or G alone (p Z 0.008). -

Use of Antitussives After the Initiation of Angiotensin-Converting Enzyme Inhibitors
저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게 l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다: 저작자표시. 귀하는 원저작자를 표시하여야 합니다. 비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다. 변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다. l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다. l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다. 저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다. Disclaimer 약학 석사학위 논문 안지오텐신 전환 효소 억제제 개시 이후 진해제의 사용 분석 Use of Antitussives After the Initiation of Angiotensin-Converting Enzyme Inhibitors 2017년 8월 서울대학교 대학원 약학과 사회약학전공 권 익 태 안지오텐신 전환 효소 억제제 개시 이후 진해제의 사용 분석 Use of Antitussives After the Initiation of Angiotensin-Converting Enzyme Inhibitors 지도교수 홍 송 희 이 논문을 권익태 석사학위논문으로 제출함 2017년 4월 서울대학교 대학원 약학과 사회약학전공 권 익 태 권익태의 석사학위논문을 인준함 2017년 6월 위 원 장 (인) 부 위 원 장 (인) 위 원 (인) Abstract Use of Antitussives After the Initiation of Angiotensin-Converting Enzyme Inhibitors Ik Tae Kwon Department of Social Pharmacy College of Pharmacy, Seoul National University Background Angiotensin-converting enzyme inhibitors (ACEI) can induce a dry cough, more frequently among Asians. If healthcare professionals fail to detect coughs induced by an ACEI, patients are at risk of getting antitussives inappropriately instead of discontinuing ACEI. The purpose of this study was to examine how the initiation of ACEI affects the likelihood of antitussive uses compared with the initiation of Angiotensin Receptor Blocker (ARB) and to determine the effect of the antitussive use on the duration and adherence of therapy in a Korean population. -

Helicidine, Un Extrait Doses May Be Given
1562 Cough Suppressants Expectorants Mucolytics and Nasal Decongestants UK preparations suggest that these doses be given up to a maxi- ◊ References. Ipecacuanha mum of 4 times daily, although in other countries higher total 1. Pons F, et al. L’effect bronchorelaxant de l’helicidine, un extrait doses may be given. d’Helix pomatia, fait intervenir une liberation de prostaglandine Hlavěnkový kořen; Ipecac; Ipecacuana; Ipécacuanha, racine d’; E2. Pathol Biol (Paris) 1999; 47: 73–80. Respiratory disorders. An FDA review of preparations avail- Ipecacuanha Root; Ipecacuanhae radix; Ipekakuána-gyökér; able over-the-counter concluded that guaifenesin was an effec- Preparations Ipekakuananjuuri (ipecacuanha root); Ipekakuanarot (ipecacuan- tive expectorant.1 The use of expectorants for productive cough Proprietary Preparations (details are given in Part 3) ha root); Ipekakuanu˛ šaknys; Korzeń ipekakuany; Raíz de ipecac- 2 uana. is discussed on p.1547. A small study found that guaifenesin Multi-ingredient: Ger.: Original Schneckensirup†. also appeared to reduce cough reflex sensitivity in patients with Ипекакуана upper respiratory-tract infections, which produce a transient in- CAS — 8012-96-2. crease in sensitivity, although it had no effect on cough reflex in ATC — R05CA04; V03AB01. Indanazoline Hydrochloride (rINNM) ⊗ healthy subjects. The mechanism for this effect was unclear. ATC Vet — QR05CA04; QV03AB01. Guaifenesin has been given to patients with altered nasal muco- Hidrocloruro de indanazolina; Indanazolin Hidroklorür; Indana- 3 Pharmacopoeias. In Eur. (see p.vii), Int., Jpn, and US. ciliary clearance associated with HIV infection. zoline, Chlorhydrate d’; Indanazolini Hydrochloridum. 1. Thomas J. Guaiphenesin—an old drug now found to be effective. Eur., Jpn, and US also include a monograph for Prepared Ipecac- Aust J Pharm 1990; 71: 101–3. -

Elif Fatma Sen BW.Indd
Use and Safety of Respiratory Medicines in Children E. F. Şen EEliflif FFatmaatma SSenen BBW.inddW.indd 1 003-01-113-01-11 115:175:17 The work presented in this thesis was conducted at the Department of Medical Informatics of the Erasmus University Medical Center, Rotterdam. The research reported in thesis was funded by the European Community’s 6th Framework Programme. Project number LSHB-CT-2005-005216: TEDDY: Task force in Europe for Drug Development for the Young. The contributions of the participating primary care physicians in the IPCI, Pedianet and IMS-DA project are greatly acknowledged. Financial support for printing this thesis was kindly provided by the department of Medical Informatics – Integrated Primary Care Information (IPCI) project of the Erasmus University Medi- cal Center; and by the J.E. Jurriaanse Stichting in Rotterdam. Cover: Optima Grafi sche Communicatie Printed by: Optima Grafi sche Communicatie Elif Fatma Şen Use and Safety of Respiratory medicines in Children ISBN: 978-94-6169-003-6 © E.F. Şen, Rotterdam, the Netherlands, 2011. All rights reserved. No part of this thesis may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without prior written permission of the holder of the copyright. EEliflif FFatmaatma SSenen BBW.inddW.indd 2 003-01-113-01-11 115:175:17 Use and Safety of Respiratory Medicines in Children Het gebruik en de bijwerkingen van respiratoire medicijnen in kinderen Proefschrift Ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnifi cus Prof.dr.