List Over Prohibited Substances and Withdrawal Times, Valid from January 1St, 2016 A. LIST of PROHIBITED SUBSTANCES
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Irina Nicoleta Cimalla Algan/Gan Sensors for Direct Monitoring Of
Irina Nicoleta Cimalla AlGaN/GaN sensors for direct monitoring of fluids and bioreactions AlGaN/GaN sensors for direct monitoring of fluids and bioreactions Irina Nicoleta Cimalla Universitätsverlag Ilmenau 2011 Impressum Bibliografische Information der Deutschen Nationalbibliothek Die Deutsche Nationalbibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliografie; detaillierte bibliografische Angaben sind im Internet über http://dnb.d-nb.de abrufbar. Diese Arbeit hat der Fakultät für Elektrotechnik und Informationstechnik der Technischen Universität Ilmenau als Dissertation vorgelegen. Tag der Einreichung: 23. November 2009 1. Gutachter: PD Dr. rer. nat. habil. Andreas Schober (Technische Universität Ilmenau) 2. Gutachter: Univ.-Prof. Dr. rer. nat. habil. Oliver Ambacher (Fraunhofer Institut für Angewandte Festkörperphysik Freiburg) 3. Gutachter: PD Dr.-Ing. habil. Frank Schwierz (Technische Universität Ilmenau) Tag der Verteidigung: 15. Juli 2010 Technische Universität Ilmenau/Universitätsbibliothek Universitätsverlag Ilmenau Postfach 10 05 65 98684 Ilmenau www.tu-ilmenau.de/universitaetsverlag Herstellung und Auslieferung Verlagshaus Monsenstein und Vannerdat OHG Am Hawerkamp 31 48155 Münster www.mv-verlag.de ISBN 978-3-86360-003-7 (Druckausgabe) URN urn:nbn:de:gbv:ilm1-2010000519 Titelfoto: photocase.com | AlexFlint „Unde dragoste nu e, nimic nu e” „Wo Liebe nicht ist, ist gar nichts“ Marin Preda: „Cel mai iubit dintre pamanteni“ 5 6 Abstract In this thesis, AlGaN/GaN heterostructures, which have shown to be reliable pH sensors, have been characterized and further developed for the in situ monitoring of cell reactions. For this reason, NG108-15 nerve cells were cultivated on sensor surfaces and their response on different neuroinhibitors was clearly monitored. First, a measurement setup for extracellular recording was designed in connection with an improved chip design and sensor technology. -

Families and the Structural Relatedness Among Globular Proteins
Protein Science (1993), 2, 884-899. Cambridge University Press. Printed in the USA. Copyright 0 1993 The Protein Society -~~ ~~~~ ~ Families and the structural relatedness among globular proteins DAVID P. YEE AND KEN A. DILL Department of Pharmaceutical Chemistry, University of California, San Francisco, California94143-1204 (RECEIVEDJanuary 6, 1993; REVISEDMANUSCRIPT RECEIVED February 18, 1993) Abstract Protein structures come in families. Are families “closely knit” or “loosely knit” entities? We describe a mea- sure of relatedness among polymer conformations. Based on weighted distance maps, this measure differs from existing measures mainly in two respects: (1) it is computationally fast, and (2) it can compare any two proteins, regardless of their relative chain lengths or degree of similarity. It does not require finding relative alignments. The measure is used here to determine the dissimilarities between all 12,403 possible pairs of 158 diverse protein structures from the Brookhaven Protein Data Bank (PDB). Combined with minimal spanning trees and hier- archical clustering methods,this measure is used to define structural families. It is also useful for rapidly searching a dataset of protein structures for specific substructural motifs.By using an analogy to distributions of Euclid- ean distances, we find that protein families are not tightly knit entities. Keywords: protein family; relatedness; structural comparison; substructure searches Pioneering work over the past 20 years has shown that positions after superposition. RMS is a useful distance proteins fall into families of related structures (Levitt & metric for comparingstructures that arenearly identical: Chothia, 1976; Richardson, 1981; Richardson & Richard- for example, when refining or comparing structures ob- son, 1989; Chothia & Finkelstein, 1990). -

Report from the 26Th Meeting on Toxinology,“Bioengineering Of
toxins Meeting Report Report from the 26th Meeting on Toxinology, “Bioengineering of Toxins”, Organized by the French Society of Toxinology (SFET) and Held in Paris, France, 4–5 December 2019 Pascale Marchot 1,* , Sylvie Diochot 2, Michel R. Popoff 3 and Evelyne Benoit 4 1 Laboratoire ‘Architecture et Fonction des Macromolécules Biologiques’, CNRS/Aix-Marseille Université, Faculté des Sciences-Campus Luminy, 13288 Marseille CEDEX 09, France 2 Institut de Pharmacologie Moléculaire et Cellulaire, Université Côte d’Azur, CNRS, Sophia Antipolis, 06550 Valbonne, France; [email protected] 3 Bacterial Toxins, Institut Pasteur, 75015 Paris, France; michel-robert.popoff@pasteur.fr 4 Service d’Ingénierie Moléculaire des Protéines (SIMOPRO), CEA de Saclay, Université Paris-Saclay, 91191 Gif-sur-Yvette, France; [email protected] * Correspondence: [email protected]; Tel.: +33-4-9182-5579 Received: 18 December 2019; Accepted: 27 December 2019; Published: 3 January 2020 1. Preface This 26th edition of the annual Meeting on Toxinology (RT26) of the SFET (http://sfet.asso.fr/ international) was held at the Institut Pasteur of Paris on 4–5 December 2019. The central theme selected for this meeting, “Bioengineering of Toxins”, gave rise to two thematic sessions: one on animal and plant toxins (one of our “core” themes), and a second one on bacterial toxins in honour of Dr. Michel R. Popoff (Institut Pasteur, Paris, France), both sessions being aimed at emphasizing the latest findings on their respective topics. Nine speakers from eight countries (Belgium, Denmark, France, Germany, Russia, Singapore, the United Kingdom, and the United States of America) were invited as international experts to present their work, and other researchers and students presented theirs through 23 shorter lectures and 27 posters. -
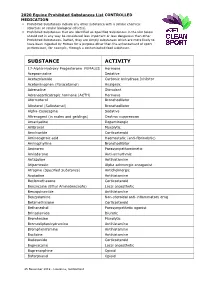
2020 Equine Prohibited Substances List CONTROLLED MEDICATION
2020 Equine Prohibited Substances List CONTROLLED MEDICATION . Prohibited Substances include any other substance with a similar chemical structure or similar biological effect(s). Prohibited Substances that are identified as Specified Substances in the List below should not in any way be considered less important or less dangerous than other Prohibited Substances. Rather, they are simply substances which are more likely to have been ingested by Horses for a purpose other than the enhancement of sport performance, for example, through a contaminated food substance. SUBSTANCE ACTIVITY 17-Alpha-Hydroxy Progesterone FEMALES Hormone Acepromazine Sedative Acetazolamide Carbonic Anhydrase Inhibitor Acetominophen (Paracetamol) Analgesic Adrenaline Stimulant Adrenocorticotropic hormone (ACTH) Hormone Aformoterol Bronchodilator Albuterol (Salbutamol) Bronchodilator Alpha-Casozepine Sedative Altrenogest (in males and geldings) Oestrus suppression Amantadine Dopaminergic Ambroxol Mucolytic Amcinonide Corticosteroid Aminocaproic acid Haemostatic (anti-fibrinolytic) Aminophylline Bronchodilator Aminorex Parasympathomimetic Amiodarone Anti-arrhythmic Antazoline Antihistamine Atipamezole Alpha adrenergic antagonist Atropine (Specified Substance) Anticholinergic Azatadine Antihistamine Beclomethasone Corticosteroid Benzocaine (Ethyl Aminobenzoate) Local anaesthetic Benzquinamide Antihistamine Benzydamine Non-steroidal anti-inflammatory drug Betamethasone Corticosteroid Bethanechol Parasympathetic agonist Brinzolamide Diuretic Bromhexine Mucolytic Bromodiphenhydramine -

National OTC Medicines List
National OTC Medicines List ‐ DraŌ 01 DRAFT National OTC Medicines List Draft 01 Ministry of Public Health of Lebanon This list was prepared under the guidance of His Excellency Minister Waêl Abou Faour andDRAFT the supervision of the Director General Dr. Walid Ammar. Editors Rita KARAM, Pharm D. PhD. Myriam WATFA, Pharm D Ghassan HAMADEH, MD.CPE FOREWORD According to the French National Agency for Medicines and Health Products Safety (ANSM), Over-the-counter (OTC) drugs are medicines that are accessible to patients in pharmacies, based on criteria set to safeguard patients’ safety. Due to their therapeutic class, these medicines could be dispensed without physician’s intervention for diagnostic, treatment initiation or maintenance purposes. Moreover, their dosage, treatment period and Package Insert Leaflet should be suitable for OTC classification. The packaging size should be in accordance with the dosage and treatment period. According to ArticleDRAFT 43 of the Law No.367 issued in 1994 related to the pharmacy practice, and the amendment of Articles 46 and 47 by Law No.91 issued in 2010, pharmacists do not have the right to dispense any medicine that is not requested by a unified prescription, unless the medicine is mentioned in a list which is established by pharmacists and physicians’ syndicates. In this regard, the Ministry of Public Health (MoPH) developed the National OTC Medicines List, and presentedit in a scientific, objective, reliable, and accessible listing. The OTC List was developed by a team of pharmacists and physicians from the Ministry of Public Health (MoPH). In order to ensure a safe and effective self- medicationat the pharmacy level, several pharmaceutical categories (e.g. -

World Journal of Biological Chemistry
ISSN 1949-8454 (online) World Journal of Biological Chemistry World J Biol Chem 2019 January 7; 10(1): 1-27 Published by Baishideng Publishing Group Inc World Journal of W J B C Biological Chemistry Contents Continuous Volume 10 Number 1 January 7, 2019 EDITORIAL 1 New findings showing how DNA methylation influences diseases Sallustio F, Gesualdo L, Gallone A MINIREVIEWS 7 Autism and carnitine: A possible link Demarquoy C, Demarquoy J 17 Last decade update for three-finger toxins: Newly emerging structures and biological activities Utkin YN WJBC https://www.wjgnet.com I January 7, 2019 Volume 10 Issue 1 World Journal of Biological Chemistry Contents Volume 10 Number 1 January 7, 2019 ABOUT COVER Editor-in-Chief of World Journal of Biological Chemistry, Vsevolod V Gurevich, PhD, Professor, Department of Pharmacology, Vanderbilt University, Nashville, TN 37232, United States AIMS AND SCOPE World Journal of Biological Chemistry (World J Biol Chem, WJBC, online ISSN 1949-8454, DOI: 10.4331) is a peer-reviewed open access academic journal that aims to guide clinical practice and improve diagnostic and therapeutic skills of clinicians. WJBC is to rapidly report the most recent developments in the research by the close collaboration of biologists and chemists in area of biochemistry and molecular biology, including: general biochemistry, pathobiochemistry, molecular and cellular biology, molecular medicine, experimental methodologies and the diagnosis, therapy, and monitoring of human disease. We encourage authors to submit their manuscripts to WJBC. We will give priority to manuscripts that are supported by major national and international foundations and those that are of great basic and clinical significance. -

Mucoactive Agents for Airway Mucus Hypersecretory Diseases
Mucoactive Agents for Airway Mucus Hypersecretory Diseases Duncan F Rogers PhD FIBiol Introduction Sputum Profile of Airway Inflammation and Mucus Hypersecretory Phenotype in Asthma, COPD, and CF Which Aspect of Airway Mucus Hypersecretion to Target? Theoretical Requirements for Effective Therapy of Airway Mucus Hypersecretion Current Recommendations for Clinical Use of Mucolytic Drugs Mucoactive Drugs N-Acetylcysteine: How Does it Work? Does it Work? Dornase Alfa Hypertonic Saline Surfactant Analysis Summary Airway mucus hypersecretion is a feature of a number of severe respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF). However, each disease has a different airway inflammatory response, with consequent, and presumably linked, mucus hypersecretory phenotype. Thus, it is possible that optimal treatment of the mucus hyper- secretory element of each disease should be disease-specific. Nevertheless, mucoactive drugs are a longstanding and popular therapeutic option, and numerous compounds (eg, N-acetylcysteine, erdosteine, and ambroxol) are available for clinical use worldwide. However, rational recommen- dation of these drugs in guidelines for management of asthma, COPD, or CF has been hampered by lack of information from well-designed clinical trials. In addition, the mechanism of action of most of these drugs is unknown. Consequently, although it is possible to categorize them according to putative mechanisms of action, as expectorants (aid and/or induce cough), mucolytics (thin -

Intensive Care Muscle Wasting and Weakness
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 862 Intensive care Muscle Wasting and Weakness Underlying Mechanisms, Muscle Specific Differences and a Specific Intervention Strategy GuiLLAumE RENAud ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 UPPSALA ISBN 978-91-554-8586-3 2013 urn:nbn:se:uu:diva-192531 Dissertation presented at Uppsala University to be publicly examined in Hedstrandsalen, Ingång 70, bv, Akademiska sjukhuset, Uppsala, Friday, March 8, 2013 at 13:15 for the degree of Doctor of Philosophy (Faculty of Medicine). The examination will be conducted in English. Abstract Renaud, G. 2013. Intensive care Muscle Wasting and Weakness: Underlying Mechanisms, Muscle Specific Differences and a Specific Intervention Strategy. Acta Universitatis Upsaliensis. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 862. 57 pp. Uppsala. ISBN 978-91-554-8586-3. The intensive care unit (ICU) condition, i.e., immobilisation, sedation and mechanical ventilation, often results in severe muscle wasting and weakness as well as a specific acquired myopathy, i.e., Acute Quadriplegic Myopathy (AQM). The exact mechanisms underlying AQM remain incomplete, but this myopathy is characterised a preferential myosin loss and a decreased muscle membrane leading to a delayed recovery from the primary disease, increased mortality and morbidity and altered quality of life of survivors. This project aims at improving our understanding of the mechanisms underlying the muscle wasting and weakness associated with AQM and explore the effects of a specific intervention strategy. Time-resolved analyses have been undertaken using a unique experimental rodent ICU model and specifically studying the muscle wasting and weakness in limb and diaphragm muscles over a two week period. -

Proteins, Peptides, and Amino Acids
Proteins, Peptides, and Amino Acids Chandra Mohan, Ph.D. Calbiochem-Novabiochem Corp., San Diego, CA The Chemical Nature of Amino Acids Peptides and polypeptides are polymers of α-amino acids. There are 20 α-amino acids that make-up all proteins of biological interest. The α-amino acids in peptides and proteins α consist of a carboxylic acid (-COOH) and an amino (-NH2) functional group attached to the same tetrahedral carbon atom. This carbon is known as the -carbon. The type of R- group attached to this carbon distinguishes one amino acid from another. Several other amino acids, also found in the body, may not be associated with peptides or proteins. These non-protein-associated amino acids perform specialized functions. Some of the α-amino acids found in proteins are also involved in other functions in the body. For example, tyrosine is involved in the formation of thyroid hormones, and glutamate and aspartate act as neurotransmitters at fast junctions. R Amino acids exist in either D- or L- enantiomorphs or stereoisomers. The D- and L-refer to the absolute confirmation of optically active compounds. With the exception of glycine, all other amino acids are mirror images that can not be superimposed. Most of the amino acids found in nature are of the L-type. Hence, eukaryotic proteins are always composed of L-amino acids although D-amino acids are found in bacterial cell walls and in some peptide antibiotics. All biological reactions occur in an aqueous phase. Hence, it is important to know how the R-group of any given amino acid dictates the structure-function relationships of peptides and proteins in solution. -

6-Bromohypaphorine from Marine Nudibranch Mollusk Hermissenda Crassicornis Is an Agonist of Human Α7 Nicotinic Acetylcholine Receptor
Mar. Drugs 2015, 13, 1255-1266; doi:10.3390/md13031255 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Article 6-Bromohypaphorine from Marine Nudibranch Mollusk Hermissenda crassicornis is an Agonist of Human α7 Nicotinic Acetylcholine Receptor Igor E. Kasheverov 1, Irina V. Shelukhina 1, Denis S. Kudryavtsev 1, Tatyana N. Makarieva 2, Ekaterina N. Spirova 1, Alla G. Guzii 2, Valentin A. Stonik 2 and Victor I. Tsetlin 1,* 1 Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Miklukho-Maklaya Street, 16/10, Moscow 117997, Russia; E-Mails: [email protected] (I.E.K.); [email protected] (I.V.S.); [email protected] (D.S.K.); [email protected] (E.N.S.) 2 G.B. Elyakov Pacific Institute of Bioorganic Chemistry (PIBOC), Russian Academy of Sciences, Prospect 100 let Vladivostoku, 159, Vladivostok 690022, Russia; E-Mails: [email protected] (T.N.M.); [email protected] (A.G.G.); [email protected] (V.A.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel./Fax: +7-495-335-57-33. Academic Editor: Alejandro M. Mayer Received: 30 December 2014 / Accepted: 15 February 2015 / Published: 12 March 2015 Abstract: 6-Bromohypaphorine (6-BHP) has been isolated from the marine sponges Pachymatisma johnstoni, Aplysina sp., and the tunicate Aplidium conicum, but data on its biological activity were not available. For the nudibranch mollusk Hermissenda crassicornis no endogenous compounds were known, and here we describe the isolation of 6-BHP from this mollusk and its effects on different nicotinic acetylcholine receptors (nAChR). -

Bromhexine Hydrochloride
Bromhexine Hydrochloride sc-204656 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Bromhexine Hydrochloride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY1 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Expectorant. Reported to change the structure of bronchial secretions and to increase the volume and reduce the viscosity of sputum. Administered in bronchitis and other respiratory conditions. Normally given by mouth. Synthetic derivative of vasicine, the active principal of Adhatoda vasica. SYNONYMS C14-H20-Br2-N2.HCl, "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "2-amino-N-cyclohexyl-3, 5-dibromo-N-methylbenzylamine hydrochloride", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", "benzenemethanamine, 2-amino-3, 5-dibromo-N-cyclohexyl-N-methyl-, ", monohydrochloride, "benzylamine, 2-amino-N- cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "benzylamine, 2-amino-N-cyclohexyl-3, 5-dibromo-N-methyl-, hydrochloride", "bromhexin hydrochloride", "N-cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", "N- cyclohexyl-N-methyl-(2-amino-3, 5-dibromobenzyl) ammonium chloride", Aletor, Bisolvomycin, Bisolvon, "Bisolvon Hydrochloride", Bromessina, Bromcilate, Bronkocin, "Dakroy Biciron", Ophtosol, "quaternary ammonium expectorant" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Harmful if swallowed. -

Drug and Medication Classification Schedule
KENTUCKY HORSE RACING COMMISSION UNIFORM DRUG, MEDICATION, AND SUBSTANCE CLASSIFICATION SCHEDULE KHRC 8-020-1 (11/2018) Class A drugs, medications, and substances are those (1) that have the highest potential to influence performance in the equine athlete, regardless of their approval by the United States Food and Drug Administration, or (2) that lack approval by the United States Food and Drug Administration but have pharmacologic effects similar to certain Class B drugs, medications, or substances that are approved by the United States Food and Drug Administration. Acecarbromal Bolasterone Cimaterol Divalproex Fluanisone Acetophenazine Boldione Citalopram Dixyrazine Fludiazepam Adinazolam Brimondine Cllibucaine Donepezil Flunitrazepam Alcuronium Bromazepam Clobazam Dopamine Fluopromazine Alfentanil Bromfenac Clocapramine Doxacurium Fluoresone Almotriptan Bromisovalum Clomethiazole Doxapram Fluoxetine Alphaprodine Bromocriptine Clomipramine Doxazosin Flupenthixol Alpidem Bromperidol Clonazepam Doxefazepam Flupirtine Alprazolam Brotizolam Clorazepate Doxepin Flurazepam Alprenolol Bufexamac Clormecaine Droperidol Fluspirilene Althesin Bupivacaine Clostebol Duloxetine Flutoprazepam Aminorex Buprenorphine Clothiapine Eletriptan Fluvoxamine Amisulpride Buspirone Clotiazepam Enalapril Formebolone Amitriptyline Bupropion Cloxazolam Enciprazine Fosinopril Amobarbital Butabartital Clozapine Endorphins Furzabol Amoxapine Butacaine Cobratoxin Enkephalins Galantamine Amperozide Butalbital Cocaine Ephedrine Gallamine Amphetamine Butanilicaine Codeine