Evaluation of Certain Food Contaminants A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS a New Methodology MASTER DISSERTATION
DM Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS A new methodology MASTER DISSERTATION João Micael da Silva Leça MASTER IN APPLIED BIOCHEMISTRY November | 2014 Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS A new methodology MASTER DISSERTATION João Micael da Silva Leça MASTER IN APPLIED BIOCHEMISTRY SUPERVISOR José Carlos Antunes Marques CO-SUPERVISOR Vanda Nulita Gomes Pereira Evolution of ethyl carbamate during Madeira wine ageing by GC-MS: a new methodology AGRADECIMENTOS Quero começar por agradecer à minha família e em especial à minha mãe, Teresa Silva, à minha avó, Teresa Teixeira, ao meu avô, Quirino Teixeira, à minha tia, Ana Teixeira, à minha madrinha, Sandra Nunes, à minha madrinha, Maria Nunes e aos meus primos Ludgero Gomes e Lília Gomes, por todo o amor e apoio que sempre me deram. Um obrigado à Fátima Spínola pelo seu amor. Um especial obrigado por toda a compreensão, pelo apoio e por todos os momentos bons que já partilhamos. Certamente seria uma pessoa muito diferente se nunca nos tivéssemos conhecido. Obrigado por tudo. Um enorme obrigado à Anabela Moura, à Manuela Godinho e ao Dr. António Godinho por toda a dedicação e apoio. O meu agradecimento torna-se difícil de expressar por palavras. E porque a vida não tem valor sem ser partilhada, um grande obrigado aos meus amigos mais próximos: Vítor Correia, Victor Andrade, Catarina Lume, João Mendes, Igor Nunes, Pedro Leme, Marco Franco, João Silva, Nuno Pestana, Válter Mendes, Julie Campbell, Luís Freitas, Igor Afonso, Roberto Aguiar, Marisa Faria, Dina Maciel, Neide Cardoso, Joaquim Lopes, Miguel Nunes, Paulo Silva, Dinarte Jesus, Hugo Câmara, Núria Fernandes, Vânia Silva, Rodolfo Silva e Ricardo Mendonça. -

Ethyl Carbamate Formation in Sub-Optimal Wine Storage Conditions and Influence of the Yeast Starter
03-larcher_05b-tomazic 26/03/13 18:21 Page65 ETHYL CARBAMATE FORMATION IN SUB-OPTIMAL WINE STORAGE CONDITIONS AND INFLUENCE OF THE YEAST STARTER Roberto LARCHER, Sergio MOSER, Ainhoa Usua MENOLLI, Loris TONIDANDEL and Giorgio NICOLINI* Unità Chimica Vitienologica e Agroalimentare, Centro Trasferimento Tecnologico - Fond. Edmund Mach, Via E. Mach 1, 38 010 San Michele all’Adige (TN), Italy Abstract Résumé Aim : To evaluate the potential risk of ethyl carbamate (EC) Objectif : Évaluer le risque potentiel de formation de carbamate formation in wine by studying its production kinetics at sub- d'éthyle (CE) dans le vin par l'étude de la cinétique de production à optimal storage temperatures. des températures de stockage sous-optimales. Methods and results : The kinetics of EC formation was Méthodes et résultats : La cinétique de formation de CE a été investigated in 60 white wines obtained from 6 varietal juices étudiée chez 60 vins blancs issus de six moûts variétaux fermentés fermented with 10 yeast strains. The wines were analysed for their avec 10 souches de levure. Les vins ont été analysés pour leur urea content at bottling, then EC formation was monitored during teneur en urée à la mise en bouteilles, puis la formation de CE a été in-bottle storage at < 12 °C for 150 days followed by 152 days at contrôlée au cours du stockage en bouteilles à < 12 °C pendant 40 °C. Storage at < 12 °C had no effect on EC formation, 150 jours, suivis par 152 jours à 40 °C. Le stockage à < 12 °C n'a regardless of initial urea content ; however, at 40 °C we found a eu aucun effet sur la formation de CE, quelle que soit la teneur en positive correlation between initial urea content and final EC urée initiale. -
![United States Patent [191 [11] Patent Number: 5,399,736 Friederichs Et Al](https://docslib.b-cdn.net/cover/4254/united-states-patent-191-11-patent-number-5-399-736-friederichs-et-al-1914254.webp)
United States Patent [191 [11] Patent Number: 5,399,736 Friederichs Et Al
USOO5399736A United States Patent [191 [11] Patent Number: 5,399,736 Friederichs et al. [45] Date of Patent: Mar. 21, 1995 [54] PROCESS FOR THE PREPARATION OF 4,369,141 1/1983 Motier ......................... 260/453P HIGHLY PURE AROMATIC DIURETHANES 4,375,000 2/1983 Merger et a1. .............. .. 560/25 AND/ OR POLYURETHANES 4,388,238 6/1983 Heitk'a'mper et a1. .. 260/239 E 4,388,246 6/ 1983 Sundermann et al. 260/453 P [75] Inventors: Wolfgang Friederichs, Cologne; 4,482,499 11/1984 Merger et a1. 260/453 P Stefan Penninger, Pulheim; Stefan 4,611,079 9/1986 Merger . .. 560/25 Wershofen, Moenchengladbach, all 4,692,550 9/1987 Engbert et a1. ................... .. 560/345 of Germany FOREIGN PATENT DOCUMENTS [73] Assignee: Bayer Aktiengesellschaft, 1458595 12/1976 United Kingdom . Leverkusen, Germany Primary Examiner-José G. Dees [21] Appl. No.: 988,659 Assistant Examiner-Samuel Barts [22] Filed: Dec. 10, 1992 Attorney, Agent, or Firm-Joseph C. Gil; N. Denise Brown [30] Foreign Application Priority Data [57] ABSTRACT Dec. 16, 1991 [DE] Germany ................... .. 41 41 402.0 This invention relates to a process for the preparation of [51] Int. Cl.6 ........... ..-. ............................ .. C07C 125/06 highly pure aromatic diurethanes and/or polyurethanes [52] US. Cl. ..................................... .. 560/25; 560/158 by reaction of the corresponding aromatic diamines [58] Field of Search ................................ .. 560/25, 158 and/or polyamines with unsubstituted carbamates with [56] References Cited the release of ammonia. The reaction is carried out in U.S. PATENT DOCUMENTS the presence of excess carbamates. The resultant reac tion mixture is freed from any solvents used, and the 2,181,663 11/1939 Martin .................................. -

EICG-Hot Spots
State of California AIR RESOURCES BOARD PUBLIC HEARING TO CONSIDER AMENDMENTS TO THE EMISSION INVENTORY CRITERIA AND GUIDELINES REPORT FOR THE AIR TOXICS “HOT SPOTS” PROGRAM STAFF REPORT: INITIAL STATEMENT OF REASONS DATE OF RELEASE: September 29, 2020 SCHEDULED FOR CONSIDERATION: November 19, 2020 Location: Please see the Public Agenda which will be posted ten days before the November 19, 2020, Board Meeting for any appropriate direction regarding a possible remote-only Board Meeting. If the meeting is to be held in person, it will be held at the California Air Resources Board, Byron Sher Auditorium, 1001 I Street, Sacramento, California 95814. This report has been reviewed by the staff of the California Air Resources Board and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Air Resources Board, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This Page Intentionally Left Blank TABLE OF CONTENTS EXECUTIVE SUMMARY .......................................................................................... 1 I. INTRODUCTION AND BACKGROUND ................................................................. 5 II. THE PROBLEM THAT THE PROPOSAL IS INTENDED TO ADDRESS .................... 6 III. BENEFITS ANTICIPATED FROM THE REGULATORY ACTION, INCLUDING THE BENEFITS OR GOALS PROVIDED IN THE AUTHORIZING STATUTE .................... 8 IV. AIR QUALITY .......................................................................................................... -

Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts
catalysts Article Environmental-Friendly Synthesis of Alkyl Carbamates from Urea and Alcohols with Silica Gel Supported Catalysts Yubo Ma 1,2 , Lei Wang 1,3,*, Xiaodong Yang 4,* and Ronghui Zhang 5 1 School of Chemical Engineering, Chongqing Chemical Industry Vocational College, Chongqing 401220, China; [email protected] 2 Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China 3 Department of Chemistry and Chemical Engineering, Xinjiang University, Urumqi 830011, China 4 Institute of Resources and Environment Science, Xinjiang University, Urumqi 830011, China 5 Guangdong Key Laboratory of Intelligent Transportation System, School of Engineering, Sun Yat-sen University, Guangzhou 510275, China; [email protected] * Correspondence: [email protected] (L.W.); [email protected] (X.Y.) Received: 7 October 2018; Accepted: 19 November 2018; Published: 23 November 2018 Abstract: TiO2/SiO2, Cr2O3-NiO/SiO2, and TiO2-Cr2O3/SiO2 were prepared by the impregnation method for alkyl carbamate synthesis using urea as the carbonyl source. Up to 97.5% methyl carbamate yield, 97% ethyl carbamate yield, and 96% butyl carbamate yield could be achieved, respectively. The catalysts were characterized by ICP-AES, BET, XRD, XPS, NH3-TPD, and EPMA. Catalytic activity investigation revealed that TiO2/SiO2, Cr2O3-NiO/SiO2, and TiO2-Cr2O3/SiO2 were effective catalysts for methyl carbamate (MC), ethyl carbamate (EC), and butyl carbamate (BC), respectively. The recycling tests suggested that these silica gel supported catalyst system is active, stable, and reusable. A total of 96–97% alkyl carbamate (methyl, ethyl, and butyl) could be obtained in a 2 L autoclave, and these data suggested that our catalyst system is relatively easy to scale up. -

EPA's Hazardous Waste Listing
Hazardous Waste Listings A User-Friendly Reference Document September 2012 Table of Contents Introduction ..................................................................................................................................... 3 Overview of the Hazardous Waste Identification Process .............................................................. 5 Lists of Hazardous Wastes .............................................................................................................. 5 Summary Chart ............................................................................................................................... 8 General Hazardous Waste Listing Resources ................................................................................. 9 § 261.11 Criteria for listing hazardous waste. .............................................................................. 11 Subpart D-List of Hazardous Wastes ............................................................................................ 12 § 261.31 Hazardous wastes from non-specific sources. ............................................................... 13 Spent solvent wastes (F001 – F005) ......................................................................................... 13 Wastes from electroplating and other metal finishing operations (F006 - F012, and F019) ... 18 Dioxin bearing wastes (F020 - F023, and F026 – F028) .......................................................... 22 Wastes from production of certain chlorinated aliphatic hydrocarbons (F024 -

Safety Data Sheet - Version 5.0 Preparation Date 4/3/2017 Latest Revision Date (If Revised) 4/13/2021 SDS Expiry Date 4/11/2024
Safety Data Sheet - Version 5.0 Preparation Date 4/3/2017 Latest Revision Date (If Revised) 4/13/2021 SDS Expiry Date 4/11/2024 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product Identifier Chemical Name Fenoxycarb Catalogue # F248870 1.2 Relevant Identified Uses of the Substance or Mixture and Uses Advised Against Product Uses To be used only for scientific research and development. Not for use in humans or animals. 1.3 Details of the Supplier of the Safety Data Sheet Company Toronto Research Chemicals 2 Brisbane Road Toronto, ON M3J 2J8 CANADA Telephone +14166659696 FAX +14166654439 Email [email protected] 1.4 Emergency Telephone Number Emergency# +1(416) 665-9696 between 0800-1700 (GMT-5) 2. HAZARDS IDENTIFICATION 2.1/2.2 Classification of the Substance or Mixture and Label Elements GHS Hazards Classification (According to EU Regulation 1272/2008 and US OSHA 1910.1200) Carcinogenicity (Category 2) Hazardous to the Aquatic Environment, Acute Hazard (Category 1) Hazardous to the Aquatic Environment, Long-Term Hazard (Category 1) GHS Hazards Identification (According to EU Regulation 1272/2008 and US OSHA 1910.1200) Signal Word Warning GHS Hazard Statements H351 Suspected of causing cancer. H400 Very toxic to aquatic life. H410 Very toxic to aquatic life with long lasting effects. GHS Precautionary Statements P273 Avoid release to the environment. P391 Collect spillage. 2.3 Unclassified Hazards/Hazards Not Otherwise Classified No data available. 3. COMPOSITION/INFORMATION ON INGREDIENTS 3.1 Substances Molecular Formula: C17H19NO4 Molecular Weight: 301.34 CAS Registry #: 72490-01-8 EC#: 276-696-7 Synonyms Insegar; Logic; Logic (growth regulator); Phenoxycarb; Ro 13-5223; ABG 6215; Eclipse; Eclipse (growth regulator); Ethyl [2-(4- phenoxyphenoxy)ethyl]carbamate; [2-(4-Phenoxyphenoxy)ethyl]carbamic Acid Ethyl Ester; N-[2-(4-Phenoxyphenoxy)ethyl] carbamic Acid Ethyl Ester; Toronto Research Chemicals - F248870 Page 1 This Safety Data Sheet contains 16 sections. -

Prioritizing Chemical Constituents in Tobacco Products and Smoke to Predict Developmental Osteotoxicity in Human Embryonic Stem Cells
California State University, San Bernardino CSUSB ScholarWorks Electronic Theses, Projects, and Dissertations Office of aduateGr Studies 12-2018 PRIORITIZING CHEMICAL CONSTITUENTS IN TOBACCO PRODUCTS AND SMOKE TO PREDICT DEVELOPMENTAL OSTEOTOXICITY IN HUMAN EMBRYONIC STEM CELLS Joseph Madrid Follow this and additional works at: https://scholarworks.lib.csusb.edu/etd Part of the Cell Biology Commons, and the Developmental Biology Commons Recommended Citation Madrid, Joseph, "PRIORITIZING CHEMICAL CONSTITUENTS IN TOBACCO PRODUCTS AND SMOKE TO PREDICT DEVELOPMENTAL OSTEOTOXICITY IN HUMAN EMBRYONIC STEM CELLS" (2018). Electronic Theses, Projects, and Dissertations. 767. https://scholarworks.lib.csusb.edu/etd/767 This Thesis is brought to you for free and open access by the Office of aduateGr Studies at CSUSB ScholarWorks. It has been accepted for inclusion in Electronic Theses, Projects, and Dissertations by an authorized administrator of CSUSB ScholarWorks. For more information, please contact [email protected]. PRIORITIZING CHEMICAL CONSTITUENTS IN TOBACCO PRODUCTS AND SMOKE TO PREDICT DEVELOPMENTAL OSTEOTOXICITY IN HUMAN EMBRYONIC STEM CELLS A Thesis Presented to the Faculty of California State University, San Bernardino In Partial Fulfillment of the Requirements for the Degree Master of Science in Biology by Joseph Valenzuela Madrid December 2018 PRIORITIZING CHEMICAL CONSTITUENTS IN TOBACCO PRODUCTS AND SMOKE TO PREDICT DEVELOPMENTAL OSTEOTOXICITY IN HUMAN EMBRYONIC STEM CELLS A Thesis Presented to the Faculty of California State University, San Bernardino by Joseph Valenzuela Madrid December 2018 Approved by: Dr. Nicole Bournias, Committee Chair, Biology Dr. Nicole zur Nieden, Committee Member, UCR, Biology Dr. Jeffrey Thompson, Committee Member © 2018 Joseph Valenzuela Madrid ABSTRACT Though it is well known that tobacco related products can cause prenatal maldevelopment, very little is known on how tobacco products affect bone tissue as it develops in the embryo. -

Epa Hazardous Waste Codes
EPA HAZARDOUS WASTE CODES Code Waste description Code Waste description D001 Ignitable waste D023 o-Cresol D002 Corrosive waste D024 m-Cresol D003 Reactive waste D025 p-Cresol D004 Arsenic D026 Cresol D005 Barium D027 1,4-Dichlorobenzene D006 Cadmium D028 1,2-Dichloroethane D007 Chromium D029 1,1-Dichloroethylene D008 Lead D030 2,4-Dinitrotoluene D009 Mercury D031 Heptachlor (and its epoxide) D010 Selenium D032 Hexachlorobenzene D011 Silver D033 Hexachlorobutadiene D012 Endrin(1,2,3,4,10,10-hexachloro-1,7-epoxy- D034 Hexachloroethane 1,4,4a,5,6,7,8,8a-octahydro-1,4-endo, endo- 5,8-dimeth-ano-naphthalene) D035 Methyl ethyl ketone D013 Lindane (1,2,3,4,5,6-hexa- D036 Nitrobenzene chlorocyclohexane, gamma isomer) D037 Pentachlorophenol D014 Methoxychlor (1,1,1-trichloro-2,2-bis [p- methoxyphenyl] ethane) D038 Pyridine D015 Toxaphene (C10 H10 Cl8, Technical D039 Tetrachloroethylene chlorinated camphene, 67-69 percent chlorine) D040 Trichlorethylene D016 2,4-D (2,4-Dichlorophenoxyacetic acid) D041 2,4,5-Trichlorophenol D017 2,4,5-TP Silvex (2,4,5- D042 2,4,6-Trichlorophenol Trichlorophenoxypropionic acid) D043 Vinyl chloride D018 Benzene D019 Carbon tetrachloride D020 Chlordane D021 Chlorobenzene D022 Chloroform B-1 EPA HAZARDOUS WASTE CODES (Continued) Code Waste description Code Waste description HAZARDOUS WASTE FROM NONSPECIFIC solvents: cresols, cresylic acid, and SOURCES nitrobenzene; and the still bottoms from the recovery of these solvents; all spent solvent F001 The following spent halogenated solvents mixtures/blends containing, before use, a used in degreasing: Tetrachloroethylene, total of ten percent or more (by volume) of trichlorethylene, methylene chloride, 1,1,1- one or more of the above nonhalogenated trichloroethane, carbon tetrachloride and solvents or those solvents listed in F001, chlorinated fluorocarbons; all spent solvent F002, and F005; and still bottoms from the mixtures/blends used in degreasing recovery of these spent solvents and spent containing, before use, a total of ten solvent mixtures. -
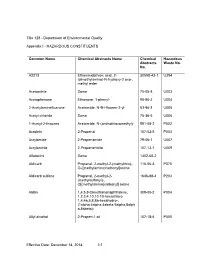
Appendix I - HAZARDOUS CONSTITUENTS
Title 128 - Department of Environmental Quality Appendix I - HAZARDOUS CONSTITUENTS Common Name Chemical Abstracts Name Chemical Hazardous Abstracts Waste No. No. A2213 Ethanimidothioic acid, 2- 30558-43-1 U394 (dimethylamino)-N-hydroxy-2 oxo-, methyl ester Acetonitrile Same 75-05-8 U003 Acetophenone Ethanone, 1-phenyl- 98-86-2 U004 2-Acetylaminefluarone Acetamide, N-9H-fluoren-2-yl- 53-96-3 U005 Acetyl chloride Same 75-36-5 U006 1-Acetyl-2-thiourea Acetamide, N-(aminothioxomethyl)- 591-08-2 P002 Acrolein 2-Propenal 107-02-8 P003 Acrylamide 2-Propenamide 79-06-1 U007 Acrylonitrile 2-Propenenitrile 107-13-1 U009 Aflatoxins Same 1402-68-2 Aldicarb Propanal, 2-methyl-2-(methylthio)-, 116-06-3 P070 O-[(methylamino)carbonyl]oxime Aldicarb sulfone Propanal, 2-methyl-2- 1646-88-4 P203 (methylsulfonyl)-, O[(methylamino)carbonyl] oxime Aldrin 1,4,5,8-Dimethanonaphthalene, 309-00-2 P004 1,2,3,4,10,10-10-hexachloro 1,4,4a,5,8,8a-hexahydro-, (1alpha,4alpha,4abeta,5alpha,8alph a,8abeta)- Allyl alcohol 2-Propen-1-ol 107-18-6 P005 Effective Date: December 14, 2014 I-1 Title 128 Appendix I Common Name Chemical Abstracts Name Chemical Hazardous Abstracts Waste No. No. Allyl chloride 1-Propane, 3-chloro 107-05-1 Aluminum phosphide Same 20859-73-8 P006 4-Aminobiphenyl [1,1'-Biphenyl]-4-amine 92-67-1 5-(Aminomethyl)-3- 3(2H)-Isoxazolone, 5-(aminomethyl) 2763-96-4 P007 isoxazolol 4-Aminopyridine 4-Pyridinamine 504-24-5 P008 Amitrole 1H-1,2,4-Triazol-3-amine 61-82-5 U011 Ammonium vanadate Vanadic acid, ammonium salt 7803-55-6 P119 Aniline Benzenamine 62-53-3 -

Ethyl Carbamate 1. Exposure Data
ETHYL CARBAMATE There appears to be no general consensus on a common trivial name for this sub- stance: ethyl carbamate and urethane (or urethan) are both commonly used; however, a preference for ethyl carbamate was noted in the more recent literature. The name urethane is also sometimes applied to high-molecular-weight polyurethanes used as foams, elastomers and coatings. Such products are not made from and do not generate the chemical ethyl carbamate on decomposition. Due to this possible confusion, the term ethyl carbamate has been used in this monograph. 1. exposure Data 1.1 Chemical and physical data 1.1.1. synonyms CAS Registry No.:.51–79–6 synonyms: Carbamic acid ethyl ester; ethylurethan; ethyl urethan; ethyl urethane; urethan; urethane 1.1.2. Chemical.formula.and.relative.molecular.mass NH2COOC2H5 Relative molecular mass: 89.1 1.1.3. Chemical.and.physical.properties.of.the.pure.substance From Budavari (2000) (a) Description: Colourless, almost odourless, columnar crystals or white granular powder; the pH of an aqueous solution is neutral (b) Boiling-point: 182–184.°C (c) Melting-point: 48–50.°C (d) solubility: Dissolves in water (1 g/0.5 mL), ethanol (1 g/0.8 mL), chloroform (1 g/0.9 mL), ether (1 g/1.5 mL), glycerol (1 g/2.5 mL) and olive oil (1 g/32 mL) (e).Volatility: Sublimes readily at 103°C at 54 mm Hg; volatile at room temperature –1281– 1282 IARC MONOGRAPHS VOLUME 96 1.1.4. Technical.products.and.impurities Trade names for ethyl carbamate include Leucothane, Leucethane and Pracarbamine.