11-261App.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Hydrogen and Carbon Isotope Fractionation During Degradation Of
ORIGINAL RESEARCH Hydrogen and carbon isotope fractionation during degradation of chloromethane by methylotrophic bacteria Thierry Nadalig1, Markus Greule2, Francßoise Bringel1,Stephane Vuilleumier1 & Frank Keppler2 1Equipe Adaptations et Interactions Microbiennes dans l’Environnement, UMR 7156 Universite de Strasbourg - CNRS, 28 rue Goethe, Strasbourg, 67083, France 2Max Planck Institute for Chemistry, Hahn-Meitner-Weg 1, 55128 Mainz, Germany Keywords Abstract Carbon isotope fractionation, chloromethane biodegradation, hydrogen isotope Chloromethane (CH3Cl) is a widely studied volatile halocarbon involved in the fractionation, methylotrophic bacteria. destruction of ozone in the stratosphere. Nevertheless, its global budget still remains debated. Stable isotope analysis is a powerful tool to constrain fluxes Correspondence of chloromethane between various environmental compartments which involve Thierry Nadalig, Universite de Strasbourg, a multiplicity of sources and sinks, and both biotic and abiotic processes. In UMR 7156 UdS-CNRS, 28 rue Goethe, this study, we measured hydrogen and carbon isotope fractionation of the 67083 Strasbourg Cedex, France. Tel: +33 3 68851973; Fax: +33 3 68852028; remaining untransformed chloromethane following its degradation by methylo- Methylobacterium extorquens Hyphomicrobium E-mail: [email protected] trophic bacterial strains CM4 and sp. MC1, which belong to different genera but both use the cmu pathway, the Funding Information only pathway for bacterial degradation of chloromethane characterized so far. Financial support for the acquisition of Hydrogen isotope fractionation for degradation of chloromethane was deter- GC-FID equipment by REALISE (http://realise. mined for the first time, and yielded enrichment factors (e)ofÀ29& and unistra.fr), the Alsace network for research À27& for strains CM4 and MC1, respectively. In agreement with previous and engineering in environmental sciences, is studies, enrichment in 13C of untransformed CH Cl was also observed, and gratefully acknowledged. -

From Acrolein Cyanohydrin
Agric. Biol. Chem., 52 (2), 589-591, 1988 589 Note carried out at iuu~zuirc under an increased pressure/' Here, we present a novel single-step synthesis of 5-(/?- methylthioethyl)hydantoin (2), in which we employed Single-step Synthesis of 5-(j6- single-step reactions of acrolein cyanohydrin (AC, 4), Methylthioethyl)hydantoin methyl mercaptan and ammoniumcarbonate in polar solvents (the AC method), and of acrolein (AL, 1), from Acrolein Cyanohydrin hydrogen cyanide, methyl mercaptan and ammonium and Acrolein carbonate (the ALmethod), accompanied with the for- mation of a-ureido-y-methylthiobutyramide (UMA, 5). Chisei Shibuya and Shunji Ouchi* By an alkaline hydrolysis of these products, dl- methionine (MT, 3) was obtained in an 85%yield on the Food Products & Pharmaceuticals Plant, bases of acrolein cyanohydrin and of acrolein. Asahi Chemical Industry Co., Ltd., Whenthe single-step hydantoination was carried out 6-2700 Asahimachi, Nobeoka, from ACor AL, a mixture of 2 and 5 was obtained. Miyazaki 882, Japan Approximately 12mol% of 5 was formed in each case of *Analytical Research Center, using AL and AC. Asahi Chemical Industry Co., Ltd., These new reactions are summarized in the following 1-3-1 Yako, Kawasaki-ku, Kawasaki-shi, equations: Kanagawa 210, Japan According to this procedure, acrolein and acrolein Received July 27, 1987 cyanohydrin, which are unstable to alkali, were not polymerized by the presence of excess ammoniumcar- bonate,-and the desired reaction proceeded in high yields. Single-step hydantoination of ACusing methanol as the A number of methods for DL-methionine synthesis solvent was carried out, and the effect of quantities of through the hydantoin intermediate have been reported methyl mercaptan, hydrogen cyanide and ammonium since Pierson1* obtained methionine in a 50%yield starting carbonate on the yield of MTwas investigated. -

Nitric Oxide in Health and Disease of the Nervous System H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3
Molecular Psychiatry (1997) 2, 300–310 1997 Stockton Press All rights reserved 1359–4184/97 $12.00 PROGRESS Nitric oxide in health and disease of the nervous system H-Y Yun1,2, VL Dawson1,3,4 and TM Dawson1,3 Departments of 1Neurology; 3Neuroscience; 4Physiology, Johns Hopkins University School of Medicine, Baltimore, MD, USA Nitric oxide (NO) is a widespread and multifunctional biological messenger molecule. It mediates vasodilation of blood vessels, host defence against infectious agents and tumors, and neurotransmission of the central and peripheral nervous systems. In the nervous system, NO is generated by three nitric oxide synthase (NOS) isoforms (neuronal, endothelial and immunologic NOS). Endothelial NOS and neuronal NOS are constitutively expressed and acti- vated by elevated intracellular calcium, whereas immunologic NOS is inducible with new RNA and protein synthesis upon immune stimulation. Neuronal NOS can be transcriptionally induced under conditions such as neuronal development and injury. NO may play a role not only in physiologic neuronal functions such as neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression but also in a variety of neurological disorders in which excessive production of NO leads to neural injury. Keywords: nitric oxide synthase; endothelium-derived relaxing factor; neurotransmission; neurotoxic- ity; neurological diseases Nitric oxide is probably the smallest and most versatile NO synthases isoforms and regulation of NO bioactive molecule identified. Convergence of multi- generation disciplinary efforts in the field of immunology, cardio- vascular pharmacology, chemistry, toxicology and neu- NO is formed by the enzymatic conversion of the guan- robiology led to the revolutionary novel concept of NO idino nitrogen of l-arginine by NO synthase (NOS). -

Cyanide Poisoning and How to Treat It Using CYANOKIT (Hydroxocobalamin for Injection) 5G
Cyanide Poisoning and How to Treat It Using CYANOKIT (hydroxocobalamin for injection) 5g 1. CYANOKIT (single 5-g vial) [package insert]. Columbia, MD: Meridian Medical Technologies, Inc.; 2011. Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT is a registered trademark of SERB Sarl, licensed by Meridian Medical Technologies, Inc., a Pfizer company. Copyright © 2015 Meridian Medical Technologies, Inc., a Pfizer company. All rights reserved. CYK783109-01 November/2015. Indication and Important Safety Information……………………………………………………………………………….………..…..3 . Identifying Cyanide Poisoning……………………………………………………………………………………………………………….…………….….5 . How CYANOKIT (hydroxocobalamin for injection) Works……………………………………………………………….12 . The Specifics of CYANOKIT…………………………………………………………………………………………………………………………….………17 . Administering CYANOKIT………………………………………………………………………………………………………………………………..……….21 . Storage and Disposal of CYANOKIT…................................................................................................................................26 . Grant Information for CYANOKIT……………………………………………………………………………………………………………………....30 . Full Prescribing Information………………………………………………………………………………………………….………………………………33 Please see Important Safety Information on slides 3-4 and full Prescribing Information for CYANOKIT starting on slide 33. CYANOKIT (hydroxocobalamin for injection) 5 g for intravenous infusion is indicated for the treatment of known or suspected cyanide poisoning. -

Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS a New Methodology MASTER DISSERTATION
DM Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS A new methodology MASTER DISSERTATION João Micael da Silva Leça MASTER IN APPLIED BIOCHEMISTRY November | 2014 Evolution of Ethyl Carbamate During Madeira Wine Ageing by GC-MS A new methodology MASTER DISSERTATION João Micael da Silva Leça MASTER IN APPLIED BIOCHEMISTRY SUPERVISOR José Carlos Antunes Marques CO-SUPERVISOR Vanda Nulita Gomes Pereira Evolution of ethyl carbamate during Madeira wine ageing by GC-MS: a new methodology AGRADECIMENTOS Quero começar por agradecer à minha família e em especial à minha mãe, Teresa Silva, à minha avó, Teresa Teixeira, ao meu avô, Quirino Teixeira, à minha tia, Ana Teixeira, à minha madrinha, Sandra Nunes, à minha madrinha, Maria Nunes e aos meus primos Ludgero Gomes e Lília Gomes, por todo o amor e apoio que sempre me deram. Um obrigado à Fátima Spínola pelo seu amor. Um especial obrigado por toda a compreensão, pelo apoio e por todos os momentos bons que já partilhamos. Certamente seria uma pessoa muito diferente se nunca nos tivéssemos conhecido. Obrigado por tudo. Um enorme obrigado à Anabela Moura, à Manuela Godinho e ao Dr. António Godinho por toda a dedicação e apoio. O meu agradecimento torna-se difícil de expressar por palavras. E porque a vida não tem valor sem ser partilhada, um grande obrigado aos meus amigos mais próximos: Vítor Correia, Victor Andrade, Catarina Lume, João Mendes, Igor Nunes, Pedro Leme, Marco Franco, João Silva, Nuno Pestana, Válter Mendes, Julie Campbell, Luís Freitas, Igor Afonso, Roberto Aguiar, Marisa Faria, Dina Maciel, Neide Cardoso, Joaquim Lopes, Miguel Nunes, Paulo Silva, Dinarte Jesus, Hugo Câmara, Núria Fernandes, Vânia Silva, Rodolfo Silva e Ricardo Mendonça. -

Hazardous Chemicals in Secondhand Marijuana Smoke
Hazardous Chemicals in Secondhand Marijuana Smoke “The following 33 marijuana smoke constituents included in Table 1 are listed under 33 Chemicals Proposition 65 as causing cancer: acetaldehyde, acetamide, acrylonitrile, 4- aminobiphenyl, arsenic, benz[a]anthracene, benzene, benzo[a]pyrene, That Can benzo[b]fluoranthene, benzo[j]fluoranthene, benzo[k]fluoranthene, benzofuran, 1,3- butadiene, cadmium, carbazole, catechol, chromium (hexavalent compounds), Cancer chrysene, dibenz[a,h]anthracene, dibenz[a,i]pyrene, dibenzo[a,e]pyrene, “Many of the chemical diethylnitrosamine, dimethylnitrosamine, formaldehyde, indeno[1,2,3,-c,d]pyrene, constituents that have been isoprene, lead, mercury, 5-methylchrysene, naphthalene, nickel, pyridine, and identified in marijuana smoke quinoline.” are carcinogens.” 2009 OEHHA document, Evidence on the Carcinogenicity of Marijuana Smoke Hydrogen Cyanide interferes with the normal use of oxygen by nearly every organ of Hydrogen the body. Exposure to hydrogen cyanide (AC) can be rapidly fatal. It has whole-body (systemic) effects, particularly affecting those organ systems most sensitive to low Cyanide oxygen levels: the central nervous system (brain), the cardiovascular system (heart Is the same chemical used for and blood vessels), and the pulmonary system (lungs). Hydrogen cyanide (AC) is a chemical weapons. chemical warfare agent (military designation, AC). Ammonia gas is a severe respiratory tract irritant. Can cause severe irritation of the Ammonia nose and throat. Can cause life-threatening accumulation of fluid in the lungs Household cleaner used on (pulmonary edema). Symptoms may include coughing, shortness of breath, difficult floors and toilets. There is 3 breathing and tightness in the chest. Symptoms may develop hours after exposure times more in secondhand and are made worse by physical effort. -

Sodium Borohydride (Nabh4) Itself Is a Relatively Mild Reducing Agent
1770 Vol. 35 (1987) Chem. Pharm. Bull. _35( 5 )1770-1776(1987). Reactions of Sodium Borohydride. IV.1) Reduction of Aromatic Sulfonyl Chlorides with Sodium Borohydride ATSUKO NOSE and TADAHIRO KUDO* Daiichi College of Pharmaceutical Sciences, 22-1 Tamagawa-cho, Minami-ku, Fukuoka 815, Japan (Received September 12, 1986) Aromatic sulfonyl chlorides were reduced with sodium borohydride in tetrahydrofuran at 0•Ž to the corresponding sulfinic acids in good yields. Further reduction proceeded when the reaction was carried out under reflux in tetrahydrofuran to give disulfide and thiophenol derivatives via sulfinic acid. Furthermore, sulfonamides were reduced with sodium borohydride by heating directly to give sulfide, disulfide and thiophenol derivatives, and diphenyl sulfone was reduced under similar conditions to give thiophenol and biphenyl. Keywords reduction; sodium borohydride; aromatic sulfonyl chloride; sulfonamide; sulfone; aromatic sulfinic acid; disulfide; sulfide; thiophenol Sodium borohydride (NaBH4) itself is a relatively mild reducing agent which is extensively used for the selective reduction of the carbonyl group of ketone, aldehyde and (carboxylic) acid halide derivatives. In the previous papers, we reported that NaBI-14 can reduce some functional groups, such as carboxylic anhydrides,2) carboxylic acids3) and nitro compounds.1) As a continuation of these studies, in the present paper, we wish to report the reduction of aromatic sulfonyl chlorides, sulfinic acids and sulfonamides with NaBH4. Many methods have been reported for the reduction of sulfonyl chlorides to sulfinic acids, such as the use of sodium sulfite,4a-d) zinc,5) electrolytic reduction,6) catalytic reduction,7) magnesium,8) sodium amalgam,9) sodium hydrogen sulfite,10) stannous chlo- TABLE I. -

Risto Laitinen/August 4, 2016 International Union of Pure and Applied Chemistry Division VIII Chemical Nomenclature and Structur
Approved Minutes, Busan 2015 Risto Laitinen/August 4, 2016 International Union of Pure and Applied Chemistry Division VIII Chemical Nomenclature and Structure Representation Approved Minutes of Division Committee Meeting in Busan, Korea, 8–9 August, 2015 1. Welcome, introductory remarks and housekeeping announcements Karl-Heinz Hellwich (KHH) welcomed everybody to the meeting, extending a special welcome to those who were attending the Division Committee meeting for the first time. He described house rules and arrangements during the meeting. KHH also regretfully reported that it has come to his attention that since the Bangor meeting in August 2014, Prof. Derek Horton (Member, Division VIII task groups on Carbohydrate and Flavonoids nomenclature; Associate Member, IUBMB-IUPAC Joint Commission on Biochemical Nomenclature) and Dr. Libuse Goebels, Member of the former Commission on Nomenclature of Organic Chemistry) have passed away. The meeting attendees paid a tribute to their memory by a moment of silence. 2. Attendance and apologies Present: Karl-Heinz Hellwich (president, KHH) , Risto Laitinen (acting secretary, RSL), Richard Hartshorn (past-president, RMH), Michael Beckett (MAB), Alan Hutton (ATH), Gerry P. Moss (GPM), Michelle Rogers (MMR), Jiří Vohlídal (JV), Andrey Yerin (AY) Observers: Leah McEwen (part time, chair of proposed project, LME), Elisabeth Mansfield (task group chair, EM), Johan Scheers (young observer, day 1; JS), Prof. Kazuyuki Tatsumi (past- president of the union, part of day 2) Apologies: Ture Damhus (secretary, TD), Vefa Ahsen, Kirill Degtyarenko, Gernot Eller, Mohammed Abul Hashem, Phil Hodge (PH), Todd Lowary, József Nagy, Ebbe Nordlander (EN), Amélia Pilar Rauter (APR), Hinnerk Rey (HR), John Todd, Lidija Varga-Defterdarović. -

Chemical Hazards Chemist Edson Haddad 2016 Sao Paulo
ENVIRONMENTAL COMPANY OF SAO PAULO STATE – CETESB REGIONAL CENTRE OF STOCKHOLM CONVENTION ON POPs FOR LATIN AMERICA AND THE CARIBBEAN REGION V INTERNATIONAL TRAINING PROGRAM ON ENVIRONMENTAL SOUND MANAGEMENT ON CHEMICALS AND WASTES, ESPECIALLY ON PERSISTENT ORGANIC POLLUTANTS (POPs) AND MERCURY (Hg) Chemical Hazards Chemist Edson Haddad 2016 Sao Paulo – SP – Brazil Safety with Chemicals • Chemicals can only be safely handled if their properties, reactions and behavior in different situations are fully known. • This knowledge allows for the selection of the appropriate PPE –Personal Protective Equipment, as well as the techniques to be employed for containment, control and environmental monitoring. Control Actions 9Neutralization, absorption, washing/dilution, soil recovery, monitoring, waste destination. I worked 20 years and had only one accident. CHEMICAL HAZARDS COFFEE WATER OXYGEN NO SUBSTANCE IS COMPLETELY FREE OF TOXIC EFFECTS TO THE BODY 12 POPs z PESTICIDES - Aldrin, dieldrin, chlordane, DDT, endrin, heptachlor, mirex, hexachlorobenzene and toxaphene; z INDUSTRIAL SUBSTANCES - PCBs (polychlorinated biphenyls) and HCB (hexachlorobenzene); z NON-INTENTIONAL SUB PRODUCTS – hexachlorobenzene; polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans (PCDD/PCDF), and PCBs. aldrin DDT mirex PCB Dioxins and furans 9 POPs • PESTICIDES - chlordecone, alpha hexachlorocyclohexane, beta hexachlorocyclohexane, lindane, pentachlorobenzene; •INDUSTRIAL SUBSTANCES - hexabromobiphenyl, hexabromodiphenyl ether and heptabromodiphenyl ether, -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -
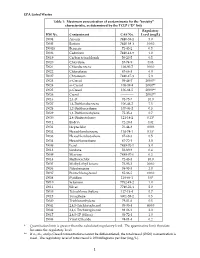
EPA Listed Wastes Table 1: Maximum Concentration of Contaminants For
EPA Listed Wastes Table 1: Maximum concentration of contaminants for the toxicity characteristic, as determined by the TCLP (D list) Regulatory HW No. Contaminant CAS No. Level (mg/L) D004 Arsenic 7440-38-2 5.0 D005 Barium 7440-39-3 100.0 D0018 Benzene 71-43-2 0.5 D006 Cadmium 7440-43-9 1.0 D019 Carbon tetrachloride 56-23-5 0.5 D020 Chlordane 57-74-9 0.03 D021 Chlorobenzene 108-90-7 100.0 D022 Chloroform 67-66-3 6.0 D007 Chromium 7440-47-3 5.0 D023 o-Cresol 95-48-7 200.0** D024 m-Cresol 108-39-4 200.0** D025 p-Cresol 106-44-5 200.0** D026 Cresol ------------ 200.0** D016 2,4-D 94-75-7 10.0 D027 1,4-Dichlorobenzene 106-46-7 7.5 D028 1,2-Dichloroethane 107-06-2 0.5 D029 1,1-Dichloroethylene 75-35-4 0.7 D030 2,4-Dinitrotoluene 121-14-2 0.13* D012 Endrin 72-20-8 0.02 D031 Heptachlor 76-44-8 0.008 D032 Hexachlorobenzene 118-74-1 0.13* D033 Hexachlorobutadiene 87-68-3 0.5 D034 Hexachloroethane 67-72-1 3.0 D008 Lead 7439-92-1 5.0 D013 Lindane 58-89-9 0.4 D009 Mercury 7439-97-6 0.2 D014 Methoxychlor 72-43-5 10.0 D035 Methyl ethyl ketone 78-93-3 200.0 D036 Nitrobenzene 98-95-3 2.0 D037 Pentachlorophenol 87-86-5 100.0 D038 Pyridine 110-86-1 5.0* D010 Selenium 7782-49-2 1.0 D011 Silver 7740-22-4 5.0 D039 Tetrachloroethylene 127-18-4 0.7 D015 Toxaphene 8001-35-2 0.5 D040 Trichloroethylene 79-01-6 0.5 D041 2,4,5-Trichlorophenol 95-95-4 400.0 D042 2,4,6-Trichlorophenol 88-06-2 2.0 D017 2,4,5-TP (Silvex) 93-72-1 1.0 D043 Vinyl Chloride 74-01-4 0.2 * Quantitation limit is greater than the calculated regulatory level.