Hickory Shad Alosa Mediocris (Mitchill) Stock Identification Using Morphometric and Meristic Characters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Traditional Ecological Knowledge in Andavadoaka, Southwest Madagascar
BLUE VENTURES CONSERVATION REPORT Josephine M. Langley Vezo Knowledge: Traditional Ecological Knowledge in Andavadoaka, southwest Madagascar 52 Avenue Road, London N6 5DR [email protected] Tel: +44 (0) 208 341 9819 Fax: +44 (0) 208 341 4821 BLUE VENTURES CONSERVATION REPORT Andavadoaka, 2006 © Blue Ventures 2006 Copyright in this publication and in all text, data and images contained herein, except as otherwise indicated, rests with Blue Ventures. Recommended citation: Langley, J. (2006). Vezo Knowledge: Traditional Ecological Knowledge in Andavadoaka, southwest Madagascar. BLUE VENTURES CONSERVATION REPORT Summary Many fisheries and marine conservation management (ii) Knowledge of marine resources is passed orally from projects throughout the world have been dogged by generation to generation failure as a result of a lack of acceptance of (iii) Traditional laws, taboos and ceremonies are commonly management interventions by local communities. used in natural resource management Evaluation of these failed studies has produced (iv) Lifestyle, food security and housing are all dependent extensive guidelines, manuals and new fields of study on natural resources and the use of coastal and marine (Bunce and Pomeroy, 2004). Community engagement, resources form an essential part of this participatory research, and promoting the use of local (v) The arrival of the Catholic Mission has reduced the knowledge have repeatedly emerged as steps necessary proportion of villagers adhering to traditional ancestor to address the problem of managing the development of worship people and their economies while simultaneously (vi) There has been a change in recent years from a barter protecting the environment (Berkes et al, 2001; Bunce and subsistence economy to a market-driven cash- based economy and Pomeroy, 2004; Wibera et al, 2004; Scholz et al, (vii) Increased income for some members of the 2004). -

Marine Biodiversity of the South East NRM Region
Marine Environment and Ecology Benthic Ecology Subprogram Marine Biodiversity of the South East NRM Region SARDI Publication No. F2009/000681-1 SARDI Research Report series No. 416 Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie SARDI Aquatic Sciences PO BOX 120 Henley Beach SA 5022 December 2009 Prepared for the Department for Environment and Heritage 1 Information Systems and Database Support Program Marine Biodiversity of the South East NRM Region Keith Rowling, Shirley Sorokin, Leonardo Mantilla and David Currie December 2009 SARDI Publication No. F2009/000681-1 SARDI Research Report Series No. 416 Prepared for the Department for Environment and Heritage 2 This Publication may be cited as: Rowling, K.P., Sorokin, S.J., Mantilla, L. & Currie, D.R.. (2009) Marine Biodiversity of the South East NRM Region. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2009/000681-1. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.sardi.sa.gov.au DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Chief of Division. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. SARDI does not accept any liability for the contents of this report or for any consequences arising from its use or any reliance placed upon it. -

Atlantic Cod (Gadus Morhua) Off Newfoundland and Labrador Determined from Genetic Variation
COSEWIC Assessment and Update Status Report on the Atlantic Cod Gadus morhua Newfoundland and Labrador population Laurentian North population Maritimes population Arctic population in Canada Newfoundland and Labrador population - Endangered Laurentian North population - Threatened Maritimes population - Special Concern Arctic population - Special Concern 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and update status report on the Atlantic cod Gadus morhua in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 76 pp. Production note: COSEWIC would like to acknowledge Jeffrey A. Hutchings for writing the update status report on the Atlantic cod Gadus morhua, prepared under contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la morue franche (Gadus morhua) au Canada Cover illustration: Atlantic Cod — Line drawing of Atlantic cod Gadus morhua by H.L. Todd. Image reproduced with permission from the Smithsonian Institution, NMNH, Division of Fishes. Her Majesty the Queen in Right of Canada, 2003 Catalogue No.CW69-14/311-2003-IN ISBN 0-662-34309-3 Recycled paper COSEWIC Assessment Summary Assessment summary — May 2003 Common name Atlantic cod (Newfoundland and Labrador population) Scientific name Gadus morhua Status Endangered Reason for designation Cod in the inshore and offshore waters of Labrador and northeastern Newfoundland, including Grand Bank, having declined 97% since the early 1970s and more than 99% since the early 1960s, are now at historically low levels. -

Fish Passage Engineering Design Criteria 2019
FISH PASSAGE ENGINEERING DESIGN CRITERIA 2019 37.2’ U.S. Fish and Wildlife Service Northeast Region June 2019 Fish and Aquatic Conservation, Fish Passage Engineering Ecological Services, Conservation Planning Assistance United States Fish and Wildlife Service Region 5 FISH PASSAGE ENGINEERING DESIGN CRITERIA June 2019 This manual replaces all previous editions of the Fish Passage Engineering Design Criteria issued by the U.S. Fish and Wildlife Service Region 5 Suggested citation: USFWS (U.S. Fish and Wildlife Service). 2019. Fish Passage Engineering Design Criteria. USFWS, Northeast Region R5, Hadley, Massachusetts. USFWS R5 Fish Passage Engineering Design Criteria June 2019 USFWS R5 Fish Passage Engineering Design Criteria June 2019 Contents List of Figures ................................................................................................................................ ix List of Tables .................................................................................................................................. x List of Equations ............................................................................................................................ xi List of Appendices ........................................................................................................................ xii 1 Scope of this Document ....................................................................................................... 1-1 1.1 Role of the USFWS Region 5 Fish Passage Engineering ............................................ -

Status of Shortnose Sturgeon in the Potomac River
Final Report Status of Shortnose Sturgeon in the Potomac River PART I – FIELD STUDIES Report prepared by: Boyd Kynard (Principal Investigator) Matthew Breece and Megan Atcheson (Project Leaders) Micah Kieffer (Co-Investigator) U. S. Geological Survey, Biological Resources Division Leetown Science Center S. O. Conte Anadromous Fish Research Center Turners Falls, Massachusetts 01376 and Mike Mangold (Assistant Project Leader) U. S. Fish and Wildlife Service, Maryland Fishery Resources Office 177 Admiral Cochrane Drive Annapolis, Maryland 21401 Report prepared for: National Park Service National Capital Region Washington, D.C USGS Natural Resources Preservation Project E 2002-7 NPS Project Coordinator – Jim Sherald BRD Project Coordinator – Ed Pendelton July 20, 2007 Gravid shortnose sturgeon female captured at river kilometer 63 on the Potomac River. Project leader, Matthew Breece (USGS), is shown with the fish on March 23, 2006. Summary Field studies during more than 3 years (March 2004–July 2007) collected data on life history of Potomac River shortnose sturgeon Acipenser brevirostrum to understand their biological status in the river. We sampled intensively for adults using gill nets, but captured only one adult in 2005. Another adult was captured in 2006 by a commercial fisher. Both fish were females with excellent body and fin condition, both had mature eggs, and both were telemetry- tagged to track their movements. The lack of capturing adults, even when intensive netting was guided by movements of tracked fish, indicated abundance of the species was less than in any river known with a sustaining population of the species. Telemetry tracking of the two females (one during September 2005–July 2007, one during March 2006–February 2007) found they remained in the river for all the year, not for just a few months like sturgeons on a coastal migration. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Final Report
FINAL REPORT REHABILITATION AND CONSERVATION THE SEAGRASS MEADOWS AT CAM HAI DONG, CAM RANH BAY, KHANH HOA PROVINCE, CENTRAL VIETNAM. Pham Huu Tri Institute of Oceanography Nhatrang, Vietnam NHATRANG, JUNE 2008 Table of Contents 1. INTRODUCTION: 3 2. OBJECTIVES: 4 3. MATERIALS AND METHODS: 4 3.1 Research methods: 4 3.2 Project sites: 4 3. 3 Duration of the project: 6 3.4 Planting techniques: 6 3.4.1 Seagrasses: 6 3.4.2 Sea-horse and sea-cucumber: 7 3.4.3 Methods of study the leaf growth rate and leaf production: 7 3.4.4 Management and taking care of the rehabilitated area at Cam Hai Dong, Cam Ranh ba: 7 4. RESULTS: 7 4.1 The natural environmental elements in the rehabilitated area – Cam Hai Dong- Cam Ranh: 7 4.2 Species diversity of seagrasses in the rehabilitated area: 8 4.3 Transplantation the Enhalus acoroides for planting: 9 4.3.1 For the shoots method: 9 4.3.2 For the seedlings methods: 11 4. 4 Results of restoration the sea-horse at the rehabilitated area: 13 4.5 Results of restoration the sea-cucumber at the rehabilitated area: 14 5. CONCLUSION: 15 6. RECOMMENDATION: 15 LITERATURE CITED: 16 APPENDIX: 17 2 1. INTRODUCTION: Khanh Hoa is the coastal province in southern central Vietnam, situated between 11°45’02’’ to 13°02’ 10’’ North latitude and from 108045’10’’to 109045’06’’ East longitude, the length of the coast lines about 300km. The shallow coastal area of Khanh Hoa province is often covered by seagrass beds especially in Cam Ranh bay area where the seagrass beds have concentrated distribution with the superficies up to more than 1000 ha, there are 6 common species of seagrasses have been found there, among them Enhalus acoroides which has long leaves (1-2 m), dense density (40- 100 shoots/m2) and high cover (70-100%) is dominant. -

Reproductive Biology of American Shad, Alosa Sapidissima, in the Mattaponi River
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2004 Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River Aaron Reid Hyle College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Oceanography Commons, and the Zoology Commons Recommended Citation Hyle, Aaron Reid, "Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River" (2004). Dissertations, Theses, and Masters Projects. Paper 1539617824. https://dx.doi.org/doi:10.25773/v5-nryc-fp96 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. REPRODUCTIVE BIOLOGY OF AMERICAN SHAD, ALOSA SAPIDISSIMA, IN THE MATTAPONI RIVER A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Aaron Reid Hyle 2004 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science A a r o i S j ^ Approved, January 2004 John EXOlney, Ph.D. Robert J. Latour, Ph.D. Herbert M. Austin, Ph.D. TABLE OF CONTENTS TABLE OF CONTENTS...................................................................... -

Report to The
Final Report to the NOAA Fisheries - Northeast Region Cooperative Research Partners Initiative on the Maine - New Hampshire Inshore Groundfish Trawl Survey July 2001 – June 2002 Final Report Fall 2001 and Spring 2002 Maine – New Hampshire Inshore Trawl Survey Submitted to the NOAA Fisheries-Northeast Region, Cooperative Research Partners Initiative (Contract 50-EANF-1-00013) By Sally A. Sherman, Vincent Manfredi, Jeanne Brown, Hannah Smith, and John Sowles Maine Department of Marine Resources Douglas E. Grout New Hampshire Fish and Game Department Donald W. Perkins, Jr. Gulf of Maine Aquarium Development Corporation And Robert Tetrault T/R Fish Inc. F/V Tara Lynn and F/V Robert Michael March 2003 Technical Research Document 03/1 TABLE OF CONTENTS Acknowledgements iii Executive Summary iv Introduction 1 Materials and Methods 3 Results 10 Fall 2001 Summary 10 Spring 2002 Summary 11 July 2001 Special Survey Summary 12 August 2001 Special Survey Summary 15 Ichthyoplankton 16 Selected Species 17 Atlantic cod (Gadus morhua) 17 Haddock (Melanogrammus aeglefinus) 20 American plaice (Hippoglossoides platessoides) 23 Yellowtail flounder (Limanda ferruginea) 25 Winter flounder (Pseudopluronectes americanus) 27 Goosefish (Lophius americanus) 30 Witch flounder (Glyptocephalus cynoglossus) 32 Atlantic herring (Clupea harengus) 34 Sea scallop (Placopecten magellanicus) 35 American lobster (Homarus americanus) 36 Discussion 39 Literature Cited 42 Appendix A: Individual Station Descriptions A-1 Appendix B: Taxa List B-1 Appendix C: Survey Catch Summaries C-1 Appendix D: Policy on Release of Trawl Survey Data D-1 ii ACKNOWLEDGEMENTS Logistically, this was a complex project that benefited from the assistance of many people. Without their help, the survey could not have been completed. -

Proceedings of the American Lobster Transferable Trap Workshop
Special Report No. 75 of the Atlantic States Marine Fisheries Commission Proceedings of the American Lobster Transferable Trap Workshop October 2002 Proceedings of the American Lobster Transferable Trap Workshop October 2002 Edited by Heather M. Stirratt Atlantic States Marine Fisheries Commission Convened by: Atlantic States Marine Fisheries Commission August 26, 2002 Washington, DC Proceedings of the American Lobster Transferable Trap Workshop ii Preface This document was prepared in cooperation with the Atlantic States Marine Fisheries Commission’s American Lobster Management Board, Technical Committee, Plan Development Team, Stock Assessment Subcommittee and the Advisory Panel. A report of the Atlantic States Marine Fisheries Commission pursuant to U.S. Department of Commerce, National Oceanic and Atmospheric Administration Award No. NA17FG2205. Proceedings of the American Lobster Transferable Trap Workshop iii Acknowledgments This report is the result of a Workshop on transferable trap programs for American lobster which was held on August 26, 2002, in Washington, DC. The workshop was convened and organized by a Workshop Steering Committee composed of: David Spencer (Advisory Panel, Chair), George Doll (New York Commercial Lobsterman, Advisory Panel Member), John Sorlien (Rhode Island Commercial Lobsterman, Advisory Panel Member), Todd Jesse (Massachusetts Commercial Lobsterman, Advisory Panel Member), Ernie Beckwith (Connecticut Department of Environmental Protection), Mark Gibson (Rhode Island Department of Environmental Management), -
Epa Contract No
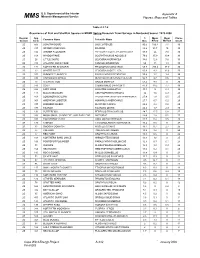
U.S. Department of the Interior Appendix A Minerals Management Service MM S Figures, Maps and Tables Table 4.2.7-6 Occurrence of Fish and Shellfish Species in MDMF Spring Research Trawl Surveys in Nantucket Sound: 1978-2008 Region/ Spp % Mean Mean Cruise Common Name Scientific Name Season Code Occ. #/Tow Wt/Tow Count 2S 503 LONGFIN SQUID LOLIGO PEALEII 90.5 100.4 7.1 30 2S 317 SPIDER CRAB UNCL MAJIDAE 88.2 93.7 10 30 2S 106 WINTER FLOUNDER PSEUDOPLEURONECTES AMERICANUS 87.9 26 7.8 30 2S 108 WINDOWPANE SCOPHTHALMUS AQUOSUS 79.6 35.9 10.4 30 2S 26 LITTLE SKATE LEUCORAJA ERINACEA 78.6 12.6 7.6 30 2S 313 ATLANTIC ROCK CRAB CANCER IRRORATUS 69 13 1.3 30 2S 171 NORTHERN SEAROBIN PRIONOTUS CAROLINUS 68.8 205.4 37.9 30 2S 23 WINTER SKATE LEUCORAJA OCELLATA 60.9 9.3 12.6 30 2S 103 SUMMER FLOUNDER PARALICHTHYS DENTATUS 55.4 1.7 1.4 30 2S 336 CHANNELED WHELK BUSYCOTYPUS CANALICULATUS 54.7 2.7 0.6 30 2S 73 ATLANTIC COD GADUS MORHUA 53.4 9.9 0 30 2S 143 SCUP STENOTOMUS CHRYSOPS 47.9 39.2 7.7 30 2S 322 LADY CRAB OVALIPES OCELLATUS 44.3 5 0.4 30 2S 141 BLACK SEA BASS CENTROPRISTIS STRIATA 30 1.6 0.9 29 2S 163 LONGHORN SCULPIN MYOXOCEPHALUS OCTODECEMSPINOSUS 27.9 1.8 0.5 25 2S 301 AMERICAN LOBSTER HOMARUS AMERICANUS 27.7 0.7 0.2 29 2S 337 KNOBBED WHELK BUSYCON CARICA 26.4 2.4 0.8 29 2S 177 TAUTOG TAUTOGA ONITIS 26.2 1.7 2.9 30 2S 131 BUTTERFISH PEPRILUS TRIACANTHUS 24.7 17.5 0.8 27 2S 338 MOON SNAIL, SHARK EYE, AND BABY-EAR NATICIDAE 24.4 1.8 0.1 27 2S 318 HORSESHOE CRAB LIMULUS POLYPHEMUS 20.7 0.4 0.5 28 2S 176 CUNNER TAUTOGOLABRUS ADSPERSUS 16.4 0.5 -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae