Feeding Behavior of American Shad During Spawning Migration in the Yo R K River, Virginia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Winter Populations, Behavior, and Seasonal Dispersal of Bald Eagles in Northwestern Illinois
WINTER POPULATIONS, BEHAVIOR, AND SEASONAL DISPERSAL OF BALD EAGLES IN NORTHWESTERN ILLINOIS WILLIAM E. SOUTHERN s a result of efforts by Bent (1937)) Broley (1952)) Herrick (1924, A 1932, and 1933), Imler (1955)) and others, numerous data on the Bald Eagle (Haliaeetus Zeucocephalus) are available. Little of the published information, however, pertains to the winter habits of the species or to winter population dynamics and seasonal movements. Between 27 November 1961 and 1 April 1962, at the Savanna Army Depot, Carroll and Jo Daviess counties, Illinois, my assistants and I spent 232 hours observing and attempting to trap eagles. Additional time was devoted to other aspects of the project (making and setting traps, securing bait, etc.). On several occasions we spent the entire daylight period in the area. The study area extended for 14 miles along the Mississippi River and its backwaters and sloughs, which were proliferated with islands (Fig. 1). Most of the slightly rolling terrain bordering the river was covered by deciduous forest. The trees along a portion of the main channel had been thinned to the point that the area (between points 1 and 2, Fig. 1) resembled a park. The study area represented only a small part of the habitat suitable for eagles near Savanna; however, it probably had the greatest abundance of food and, there- fore, eagles (we observed only four outside the Depot; three of these during spring dispersal). The concentrations reported during our study were larger than those reported elsewhere in the state. My objectives were to record behavior, live-trap, color-mark, and determine the movements of Bald Eagles during the winter and early spring. -

Shad in the Schools, NC
Attachment 1 Food Web Activity Student Sheet Background Information: Shad in the Schools, N.C. The coastal rivers and shallow marine environments along the coast of North Carolina are home to complex ecosystems. Within these freshwater and saltwater ecosystems there is an intimate relationship between many different producers and consumers. These produces and consumers create an interconnected food web. Each organism is dependent on clean water, oxygen, and healthy riparian corridors on the lands adjacent to these waters. The disruption of just one component of any food web can disturb all living things, including humans. American shad, a member of the herring family, is one species of fish that plays a critical role in the river and near shore shallow marine ecosystems of North Carolina. Shad are an anadromous species, meaning they live in the ocean but return to the rivers of North Carolina in the spring to spawn, or reproduce, each spring. This means that shad serve as a food source for many animals living in both inland freshwater and marine environments. Predators of shad include other fishes such as striped bass and king mackerel, bald eagles, bottlenose dolphin, and humans. Bald eagles have evolved to raise their young at the same time that anadromous fishes, such as shad, return to the rivers to spawn so that the young eaglets will have a plentiful food source. This evolutionary trait highlights the connectivity that can be found within food webs. Bald Eagles can be viewed now through a new technology, web cams. To see eagles that feed on herring near North Carolina, view http://www.friendsofblackwater.org/camhtm2.html. -

Atlantic Cod (Gadus Morhua) Off Newfoundland and Labrador Determined from Genetic Variation
COSEWIC Assessment and Update Status Report on the Atlantic Cod Gadus morhua Newfoundland and Labrador population Laurentian North population Maritimes population Arctic population in Canada Newfoundland and Labrador population - Endangered Laurentian North population - Threatened Maritimes population - Special Concern Arctic population - Special Concern 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and update status report on the Atlantic cod Gadus morhua in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 76 pp. Production note: COSEWIC would like to acknowledge Jeffrey A. Hutchings for writing the update status report on the Atlantic cod Gadus morhua, prepared under contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la morue franche (Gadus morhua) au Canada Cover illustration: Atlantic Cod — Line drawing of Atlantic cod Gadus morhua by H.L. Todd. Image reproduced with permission from the Smithsonian Institution, NMNH, Division of Fishes. Her Majesty the Queen in Right of Canada, 2003 Catalogue No.CW69-14/311-2003-IN ISBN 0-662-34309-3 Recycled paper COSEWIC Assessment Summary Assessment summary — May 2003 Common name Atlantic cod (Newfoundland and Labrador population) Scientific name Gadus morhua Status Endangered Reason for designation Cod in the inshore and offshore waters of Labrador and northeastern Newfoundland, including Grand Bank, having declined 97% since the early 1970s and more than 99% since the early 1960s, are now at historically low levels. -

Volume III, Chapter 6 American Shad
Volume III, Chapter 6 American Shad TABLE OF CONTENTS 6.0 American Shad (Alosa sapidissima) ........................................................................... 6-1 6.1 Introduction................................................................................................................. 6-1 6.2 Life History & Requirements...................................................................................... 6-1 6.2.1 Spawning Conditions ........................................................................................... 6-2 6.2.2 Incubation ............................................................................................................ 6-2 6.2.3 Larvae & Juveniles .............................................................................................. 6-2 6.2.4 Adult..................................................................................................................... 6-2 6.2.5 Movements in Fresh Water.................................................................................. 6-3 6.2.6 Ocean Migration.................................................................................................. 6-4 6.3 Population Identification & Distribution .................................................................... 6-4 6.3.1 Life History Differences....................................................................................... 6-4 6.3.2 Genetic Differences.............................................................................................. 6-4 6.4 Status & Abundance -

Status of Shortnose Sturgeon in the Potomac River
Final Report Status of Shortnose Sturgeon in the Potomac River PART I – FIELD STUDIES Report prepared by: Boyd Kynard (Principal Investigator) Matthew Breece and Megan Atcheson (Project Leaders) Micah Kieffer (Co-Investigator) U. S. Geological Survey, Biological Resources Division Leetown Science Center S. O. Conte Anadromous Fish Research Center Turners Falls, Massachusetts 01376 and Mike Mangold (Assistant Project Leader) U. S. Fish and Wildlife Service, Maryland Fishery Resources Office 177 Admiral Cochrane Drive Annapolis, Maryland 21401 Report prepared for: National Park Service National Capital Region Washington, D.C USGS Natural Resources Preservation Project E 2002-7 NPS Project Coordinator – Jim Sherald BRD Project Coordinator – Ed Pendelton July 20, 2007 Gravid shortnose sturgeon female captured at river kilometer 63 on the Potomac River. Project leader, Matthew Breece (USGS), is shown with the fish on March 23, 2006. Summary Field studies during more than 3 years (March 2004–July 2007) collected data on life history of Potomac River shortnose sturgeon Acipenser brevirostrum to understand their biological status in the river. We sampled intensively for adults using gill nets, but captured only one adult in 2005. Another adult was captured in 2006 by a commercial fisher. Both fish were females with excellent body and fin condition, both had mature eggs, and both were telemetry- tagged to track their movements. The lack of capturing adults, even when intensive netting was guided by movements of tracked fish, indicated abundance of the species was less than in any river known with a sustaining population of the species. Telemetry tracking of the two females (one during September 2005–July 2007, one during March 2006–February 2007) found they remained in the river for all the year, not for just a few months like sturgeons on a coastal migration. -

Humboldt Bay Fishes
Humboldt Bay Fishes ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> ·´¯`·._.·´¯`·.. ><((((º>`·._ .·´¯`·. _ .·´¯`·. ><((((º> Acknowledgements The Humboldt Bay Harbor District would like to offer our sincere thanks and appreciation to the authors and photographers who have allowed us to use their work in this report. Photography and Illustrations We would like to thank the photographers and illustrators who have so graciously donated the use of their images for this publication. Andrey Dolgor Dan Gotshall Polar Research Institute of Marine Sea Challengers, Inc. Fisheries And Oceanography [email protected] [email protected] Michael Lanboeuf Milton Love [email protected] Marine Science Institute [email protected] Stephen Metherell Jacques Moreau [email protected] [email protected] Bernd Ueberschaer Clinton Bauder [email protected] [email protected] Fish descriptions contained in this report are from: Froese, R. and Pauly, D. Editors. 2003 FishBase. Worldwide Web electronic publication. http://www.fishbase.org/ 13 August 2003 Photographer Fish Photographer Bauder, Clinton wolf-eel Gotshall, Daniel W scalyhead sculpin Bauder, Clinton blackeye goby Gotshall, Daniel W speckled sanddab Bauder, Clinton spotted cusk-eel Gotshall, Daniel W. bocaccio Bauder, Clinton tube-snout Gotshall, Daniel W. brown rockfish Gotshall, Daniel W. yellowtail rockfish Flescher, Don american shad Gotshall, Daniel W. dover sole Flescher, Don stripped bass Gotshall, Daniel W. pacific sanddab Gotshall, Daniel W. kelp greenling Garcia-Franco, Mauricio louvar -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Reproductive Biology of American Shad, Alosa Sapidissima, in the Mattaponi River
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2004 Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River Aaron Reid Hyle College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Oceanography Commons, and the Zoology Commons Recommended Citation Hyle, Aaron Reid, "Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River" (2004). Dissertations, Theses, and Masters Projects. Paper 1539617824. https://dx.doi.org/doi:10.25773/v5-nryc-fp96 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. REPRODUCTIVE BIOLOGY OF AMERICAN SHAD, ALOSA SAPIDISSIMA, IN THE MATTAPONI RIVER A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Aaron Reid Hyle 2004 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science A a r o i S j ^ Approved, January 2004 John EXOlney, Ph.D. Robert J. Latour, Ph.D. Herbert M. Austin, Ph.D. TABLE OF CONTENTS TABLE OF CONTENTS...................................................................... -

Report to The
Final Report to the NOAA Fisheries - Northeast Region Cooperative Research Partners Initiative on the Maine - New Hampshire Inshore Groundfish Trawl Survey July 2001 – June 2002 Final Report Fall 2001 and Spring 2002 Maine – New Hampshire Inshore Trawl Survey Submitted to the NOAA Fisheries-Northeast Region, Cooperative Research Partners Initiative (Contract 50-EANF-1-00013) By Sally A. Sherman, Vincent Manfredi, Jeanne Brown, Hannah Smith, and John Sowles Maine Department of Marine Resources Douglas E. Grout New Hampshire Fish and Game Department Donald W. Perkins, Jr. Gulf of Maine Aquarium Development Corporation And Robert Tetrault T/R Fish Inc. F/V Tara Lynn and F/V Robert Michael March 2003 Technical Research Document 03/1 TABLE OF CONTENTS Acknowledgements iii Executive Summary iv Introduction 1 Materials and Methods 3 Results 10 Fall 2001 Summary 10 Spring 2002 Summary 11 July 2001 Special Survey Summary 12 August 2001 Special Survey Summary 15 Ichthyoplankton 16 Selected Species 17 Atlantic cod (Gadus morhua) 17 Haddock (Melanogrammus aeglefinus) 20 American plaice (Hippoglossoides platessoides) 23 Yellowtail flounder (Limanda ferruginea) 25 Winter flounder (Pseudopluronectes americanus) 27 Goosefish (Lophius americanus) 30 Witch flounder (Glyptocephalus cynoglossus) 32 Atlantic herring (Clupea harengus) 34 Sea scallop (Placopecten magellanicus) 35 American lobster (Homarus americanus) 36 Discussion 39 Literature Cited 42 Appendix A: Individual Station Descriptions A-1 Appendix B: Taxa List B-1 Appendix C: Survey Catch Summaries C-1 Appendix D: Policy on Release of Trawl Survey Data D-1 ii ACKNOWLEDGEMENTS Logistically, this was a complex project that benefited from the assistance of many people. Without their help, the survey could not have been completed. -

American Shad at Home American Shad-Themed Activities for Students and Their Caregivers to Complete at Home
American Shad at Home American shad-themed activities for students and their caregivers to complete at home. Early Learners (K-2nd) 1. American shad are a type of fish! But what is a fish, and what things do all fish have in common? Visit this webpage from National Geographic to learn more about fish and spend time looking closely at the photographs of the different kinds of fish. Make a list of the things that fish have in common. For example, do all fish have fins? Next, make a list of all the things you and fish have in common. For example, do fish have eyes? What about bones? You can create your lists by writing words or drawing pictures. 2. Birds like bald eagles and osprey hunt for fish, including American shad! Can you help this eagle find fish by completing this maze? If you have a printer at home, you can print the maze. If you don’t, you can use your finger to trace the route the eagle should take on a computer, tablet, or phone screen. 3. American shad travel long distances throughout their life – from the rivers where they were born, out to the ocean, and back again! Shad may encounter many obstacles on their journey: predators like dolphins and eagles that want to eat them, dams and locks that prevent them from getting where they need to go, rough water and strong currents that make it hard to swim, etc. Create your own obstacle course at home using materials you already have: sheets, pillows, blankets, blocks, cardboard boxes, etc. -
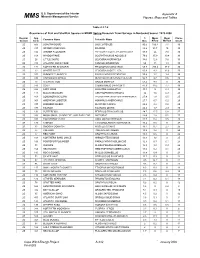
Epa Contract No
U.S. Department of the Interior Appendix A Minerals Management Service MM S Figures, Maps and Tables Table 4.2.7-6 Occurrence of Fish and Shellfish Species in MDMF Spring Research Trawl Surveys in Nantucket Sound: 1978-2008 Region/ Spp % Mean Mean Cruise Common Name Scientific Name Season Code Occ. #/Tow Wt/Tow Count 2S 503 LONGFIN SQUID LOLIGO PEALEII 90.5 100.4 7.1 30 2S 317 SPIDER CRAB UNCL MAJIDAE 88.2 93.7 10 30 2S 106 WINTER FLOUNDER PSEUDOPLEURONECTES AMERICANUS 87.9 26 7.8 30 2S 108 WINDOWPANE SCOPHTHALMUS AQUOSUS 79.6 35.9 10.4 30 2S 26 LITTLE SKATE LEUCORAJA ERINACEA 78.6 12.6 7.6 30 2S 313 ATLANTIC ROCK CRAB CANCER IRRORATUS 69 13 1.3 30 2S 171 NORTHERN SEAROBIN PRIONOTUS CAROLINUS 68.8 205.4 37.9 30 2S 23 WINTER SKATE LEUCORAJA OCELLATA 60.9 9.3 12.6 30 2S 103 SUMMER FLOUNDER PARALICHTHYS DENTATUS 55.4 1.7 1.4 30 2S 336 CHANNELED WHELK BUSYCOTYPUS CANALICULATUS 54.7 2.7 0.6 30 2S 73 ATLANTIC COD GADUS MORHUA 53.4 9.9 0 30 2S 143 SCUP STENOTOMUS CHRYSOPS 47.9 39.2 7.7 30 2S 322 LADY CRAB OVALIPES OCELLATUS 44.3 5 0.4 30 2S 141 BLACK SEA BASS CENTROPRISTIS STRIATA 30 1.6 0.9 29 2S 163 LONGHORN SCULPIN MYOXOCEPHALUS OCTODECEMSPINOSUS 27.9 1.8 0.5 25 2S 301 AMERICAN LOBSTER HOMARUS AMERICANUS 27.7 0.7 0.2 29 2S 337 KNOBBED WHELK BUSYCON CARICA 26.4 2.4 0.8 29 2S 177 TAUTOG TAUTOGA ONITIS 26.2 1.7 2.9 30 2S 131 BUTTERFISH PEPRILUS TRIACANTHUS 24.7 17.5 0.8 27 2S 338 MOON SNAIL, SHARK EYE, AND BABY-EAR NATICIDAE 24.4 1.8 0.1 27 2S 318 HORSESHOE CRAB LIMULUS POLYPHEMUS 20.7 0.4 0.5 28 2S 176 CUNNER TAUTOGOLABRUS ADSPERSUS 16.4 0.5 -

American Shad Life History
American Shad Life History, (when and where do they spawn, where do they live, what do they eat, what are their predators , how old do they get, where do they go, how do we know?) How do we know all this about American Shad? Answer = Mostly from college students (students doing research as part of their educational requirements for fishery science graduate degrees at universities in North Carolina. Above are graduate students from NCSU and on the left is a professor at East Carolina University who has taught many students with research projects Above are nets that can be as well. lowered off bridges to collect eggs and larvae from the water Dr. Joe Hightower at NCSU has had several students that have contributed to as the current flows under the understanding American shad in our rivers and Dr. Roger Rulifson has had Bridge. students as well that contributed greatly to our understanding of American shad movement patterns. This is a great situation because it helps educate students and helps them develop work experience that makes it easier for them to find jobs. A How do we know where they go? American shad with dart tag with identification numbers. Tagging fish allowed biologists to learn where American shad lived at different times. More recently some fish have been tagged with implanted transponders that can be detected by remote sensors placed along the rivers. Transponder tags are also used by veterinarians in dogs and cats so that if you lose your pet the pet’s information owner / address are always with the pet in case they are found, but have lost their collar tags.