Status of Shortnose Sturgeon in the Potomac River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sturgeons of the Caspian Sea and Ural River
Photo and image credits: Front Cover: Top left, beluga sturgeon (Huso huso); Top Right: Russian sturgeon (Acipenser gueldenstaedtii); Bottom: stellate sturgeon (Acipenser stellatus); Todd Stailey, Tennessee Aquarium. Back Cover: “FISH IS OUR TREASURE”, Phaedra Doukakis, Ph. D. IOCS; Page 1: Shannon Crownover, The Nature Conservancy; Page 6, 9, 10, 11: Phaedra Doukakis, Ph. D., IOCS; Page 4, 5, 7 designed by Grace Lewis. Brochure content was developed by Alison Ormsby, Ph.D. and Phaedra Doukakis, Ph. D. with editorial review by Yael Wyner, Ph. D. Layout was designed by Grace Lewis. The brochure was developed under a generous grant from Agip KCO. Agip KCO Tel.: international line: 1, K. Smagulov Street +39 02 9138 3300 Atyrau, 06002 Tel: local lines: Republic of Kazakhstan (+7) 7122 92 3300 Fax: (+7) 7122 92 3310 Designed and printed in Kazakhstan www.agipkco.com Sturgeons of the Caspian Sea and Ural River www.oceanconservationscience.org A Unique and Precious Resource What are Sturgeon? River, other rivers off the Caspian Sea are used by sturgeons for With bony plates called scutes on their bodies and ancestors that reproduction, including the Volga River in Russia and the Kura date to the time of dinosaurs, sturgeons are unusual fish. Unlike River in Azerbaijan. However, dams on the Volga and Kura have other types of fish, sturgeons have scutes instead of scales. blocked sturgeons from being able to migrate upriver, and have changed the quality of the rivers so they are no longer able to support sturgeon reproduction. Reproduction: For sturgeon, the process of mixing female eggs and male sperm to create a fertilized egg that hatches into a baby sturgeon. -

Sport-Fish-Identification.Pdf
Walleye Walleye have two distinct fins on their back, the first with large spines. Lake Sturgeon They have a yellow-olive back, brassy, silvery sides with yellow spots, a white underside, and white on the lower lobe of the tail. Dusky vertical Lake Sturgeon are a Threatened Species due to population size and bars are often found on the body as well. concerns with viability. Lake Sturgeon have a large brown or grey body covered with tough, leather- like tissue and five rows of bony plates. They have a shark-like, upturned tail and a pointed snout with four barbels. Sauger Lake Whitefish are olive-green to blue on the back, with silvery sides.They Sauger are a Threatened Species due to hybridization, habitat Lakehave a small Whitefish mouth below a rounded snout, and a deeply forked tail. degradation and overharvest. Sauger are golden olive on the back with silver-yellow sides and a white underside. They also have a large spiny dorsal fin, distinct rows of spots on the dorsal fins and three or four dusky vertical bars on the body. Mountain Whitefish have large scales, no spots and small mouths with no Burbot Mountainteeth. Their general Whitefish body colour is a bronze-white or greenish white. Burbot have a slim, brownish black body with smooth skin, a flattened head, and a fin that stretches along the back half of the body. Distinctive barbels hang from the lower jaw and nostrils. Goldeye Northern Pike Goldeye have prominent eyes with bright yellow pupils, a blunt head, and Northern Pike are a long, slender fish with duck-like jaws and a long, flat a deep, compressed body. -

Atlantic Cod (Gadus Morhua) Off Newfoundland and Labrador Determined from Genetic Variation
COSEWIC Assessment and Update Status Report on the Atlantic Cod Gadus morhua Newfoundland and Labrador population Laurentian North population Maritimes population Arctic population in Canada Newfoundland and Labrador population - Endangered Laurentian North population - Threatened Maritimes population - Special Concern Arctic population - Special Concern 2003 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2003. COSEWIC assessment and update status report on the Atlantic cod Gadus morhua in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 76 pp. Production note: COSEWIC would like to acknowledge Jeffrey A. Hutchings for writing the update status report on the Atlantic cod Gadus morhua, prepared under contract with Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Rapport du COSEPAC sur la situation de la morue franche (Gadus morhua) au Canada Cover illustration: Atlantic Cod — Line drawing of Atlantic cod Gadus morhua by H.L. Todd. Image reproduced with permission from the Smithsonian Institution, NMNH, Division of Fishes. Her Majesty the Queen in Right of Canada, 2003 Catalogue No.CW69-14/311-2003-IN ISBN 0-662-34309-3 Recycled paper COSEWIC Assessment Summary Assessment summary — May 2003 Common name Atlantic cod (Newfoundland and Labrador population) Scientific name Gadus morhua Status Endangered Reason for designation Cod in the inshore and offshore waters of Labrador and northeastern Newfoundland, including Grand Bank, having declined 97% since the early 1970s and more than 99% since the early 1960s, are now at historically low levels. -

Lake Superior Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake Superior Food Web MENT OF C Sea Lamprey Walleye Burbot Lake Trout Chinook Salmon Brook Trout Rainbow Trout Lake Whitefish Bloater Yellow Perch Lake herring Rainbow Smelt Deepwater Sculpin Kiyi Ruffe Lake Sturgeon Mayfly nymphs Opossum Shrimp Raptorial waterflea Mollusks Amphipods Invasive waterflea Chironomids Zebra/Quagga mussels Native waterflea Calanoids Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake Superior, 2009. 734-741-2235 - www.glerl.noaa.gov Lake Superior Food Web Sea Lamprey Macroinvertebrates Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Chironomids/Oligochaetes. Larval insects and worms that live on the lake fastens onto its prey and rasps out a hole with its rough tongue. bottom. Feed on detritus. Species present are a good indicator of water quality. Piscivores (Fish Eaters) Amphipods (Diporeia). The most common species of amphipod found in fish diets that began declining in the late 1990’s. Chinook salmon (Oncorhynchus tshawytscha). Pacific salmon species stocked as a trophy fish and to control alewife. Opossum shrimp (Mysis relicta). An omnivore that feeds on algae and small cladocerans. -

Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs
little fish BIG IMPACT Managing a crucial link in ocean food webs A report from the Lenfest Forage Fish Task Force The Lenfest Ocean Program invests in scientific research on the environmental, economic, and social impacts of fishing, fisheries management, and aquaculture. Supported research projects result in peer-reviewed publications in leading scientific journals. The Program works with the scientists to ensure that research results are delivered effectively to decision makers and the public, who can take action based on the findings. The program was established in 2004 by the Lenfest Foundation and is managed by the Pew Charitable Trusts (www.lenfestocean.org, Twitter handle: @LenfestOcean). The Institute for Ocean Conservation Science (IOCS) is part of the Stony Brook University School of Marine and Atmospheric Sciences. It is dedicated to advancing ocean conservation through science. IOCS conducts world-class scientific research that increases knowledge about critical threats to oceans and their inhabitants, provides the foundation for smarter ocean policy, and establishes new frameworks for improved ocean conservation. Suggested citation: Pikitch, E., Boersma, P.D., Boyd, I.L., Conover, D.O., Cury, P., Essington, T., Heppell, S.S., Houde, E.D., Mangel, M., Pauly, D., Plagányi, É., Sainsbury, K., and Steneck, R.S. 2012. Little Fish, Big Impact: Managing a Crucial Link in Ocean Food Webs. Lenfest Ocean Program. Washington, DC. 108 pp. Cover photo illustration: shoal of forage fish (center), surrounded by (clockwise from top), humpback whale, Cape gannet, Steller sea lions, Atlantic puffins, sardines and black-legged kittiwake. Credits Cover (center) and title page: © Jason Pickering/SeaPics.com Banner, pages ii–1: © Brandon Cole Design: Janin/Cliff Design Inc. -

Reproductive Biology of American Shad, Alosa Sapidissima, in the Mattaponi River
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 2004 Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River Aaron Reid Hyle College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Oceanography Commons, and the Zoology Commons Recommended Citation Hyle, Aaron Reid, "Reproductive Biology of American Shad, Alosa sapidissima, in the Mattaponi River" (2004). Dissertations, Theses, and Masters Projects. Paper 1539617824. https://dx.doi.org/doi:10.25773/v5-nryc-fp96 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. REPRODUCTIVE BIOLOGY OF AMERICAN SHAD, ALOSA SAPIDISSIMA, IN THE MATTAPONI RIVER A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment Of the Requirements for the Degree of Master of Science by Aaron Reid Hyle 2004 APPROVAL SHEET This thesis is submitted in partial fulfillment of The requirements for the degree of Master of Science A a r o i S j ^ Approved, January 2004 John EXOlney, Ph.D. Robert J. Latour, Ph.D. Herbert M. Austin, Ph.D. TABLE OF CONTENTS TABLE OF CONTENTS...................................................................... -

Report to The
Final Report to the NOAA Fisheries - Northeast Region Cooperative Research Partners Initiative on the Maine - New Hampshire Inshore Groundfish Trawl Survey July 2001 – June 2002 Final Report Fall 2001 and Spring 2002 Maine – New Hampshire Inshore Trawl Survey Submitted to the NOAA Fisheries-Northeast Region, Cooperative Research Partners Initiative (Contract 50-EANF-1-00013) By Sally A. Sherman, Vincent Manfredi, Jeanne Brown, Hannah Smith, and John Sowles Maine Department of Marine Resources Douglas E. Grout New Hampshire Fish and Game Department Donald W. Perkins, Jr. Gulf of Maine Aquarium Development Corporation And Robert Tetrault T/R Fish Inc. F/V Tara Lynn and F/V Robert Michael March 2003 Technical Research Document 03/1 TABLE OF CONTENTS Acknowledgements iii Executive Summary iv Introduction 1 Materials and Methods 3 Results 10 Fall 2001 Summary 10 Spring 2002 Summary 11 July 2001 Special Survey Summary 12 August 2001 Special Survey Summary 15 Ichthyoplankton 16 Selected Species 17 Atlantic cod (Gadus morhua) 17 Haddock (Melanogrammus aeglefinus) 20 American plaice (Hippoglossoides platessoides) 23 Yellowtail flounder (Limanda ferruginea) 25 Winter flounder (Pseudopluronectes americanus) 27 Goosefish (Lophius americanus) 30 Witch flounder (Glyptocephalus cynoglossus) 32 Atlantic herring (Clupea harengus) 34 Sea scallop (Placopecten magellanicus) 35 American lobster (Homarus americanus) 36 Discussion 39 Literature Cited 42 Appendix A: Individual Station Descriptions A-1 Appendix B: Taxa List B-1 Appendix C: Survey Catch Summaries C-1 Appendix D: Policy on Release of Trawl Survey Data D-1 ii ACKNOWLEDGEMENTS Logistically, this was a complex project that benefited from the assistance of many people. Without their help, the survey could not have been completed. -

Fisheries, Midwest Region Conserving America's Fisheries
Fisheries | U.S. Fish and Wildlife Service Midwest Region Fisheries, Midwest Region Conserving America's Fisheries Subscribe Now! May 21, 2016 - World Fish Migration Day! Field Focus Neosho National Fish Hatchery Study Reveals Patterns of Lake Trout Things at the Neosho National Fish Hatchery (NFH) have been heating up Spring Loaded! lately, in more ways than one. We’ve been busy with our sturgeon, mussels...Read More Lake Sturgeon...They’re Doing Well! Current Edition PDF May 21, 2016 - World Fish Migration Day! Removing Barriers in the Marengo River Have fun while learning and creating awareness about some of the United States’ cool migratory fishes...Read More Fish Tails Field Notes "Fish Tails” refers to articles that are submitted by "Field Notes” is an online searchable database that field staff that do not appear as a feature in the current showcases hundreds of employee-written summaries edition of Fish Lines. These articles provide examples of field activities and accomplishments of the U.S. Fish of the diverse work that the Service's Midwest Fisheries and Wildlife Service from across the nation. Program and partners perform on behalf of our aquatic resources and for the benefit of the American public. Archive 2016 2015 2014 2013 2012 2011 2010 2009 Editorial Staff Tim Smigielski, Editor Karla Bartelt, Webmaster Last updated: May 19, 2016 U.S. Fish and Wildlife Service Home Page | Department of the Interior | USA.gov | About the U.S. Fish and Wildlife Service | Accessibility | Privacy | Notices | Disclaimer | FOIA http://www.fws.gov/midwest/fisheries/fishlines/index.html[5/19/2016 12:11:31 PM] Fisheries | U.S. -

NORTH AMERICAN GREEN STURGEON (Acipenser Medirostris) AS an ENDANGERED OR THREATENED SPECIES UNDER the ENDANGERED SPECIES ACT
PETITION TO LIST THE NORTH AMERICAN GREEN STURGEON (Acipenser medirostris) AS AN ENDANGERED OR THREATENED SPECIES UNDER THE ENDANGERED SPECIES ACT ENVIRONMENTAL PROTECTION INFORMATION CENTER CENTER FOR BIOLOGICAL DIVERSITY WATERKEEPERS NORTHERN CALIFORNIA PETITIONERS JUNE 2001 NOTICE OF PETITION Environmental Protection Information Center P.O. Box 397 Garberville, CA 95542 (707) 923-2931 Contact: Cynthia Elkins Center for Biological Diversity P.O. Box 40090 Berkeley, CA 94704 (510) 841-0812 Contact: Jeff Miller WaterKeepers Northern California Presidio Building 1004 San Francisco, CA 94129 (415) 561.2299 ext. 14 Contact: Jonathan Kaplan Petitioners Environmental Protection Information Center (“EPIC”), Center for Biological Diversity (“CBD”), and WaterKeepers Northern California (“WaterKeepers”) formally request that the National Marine Fisheries Service (“NMFS”) list the North American green sturgeon (Acipenser medirostris) as an endangered species under the federal Endangered Species Act (“ESA”), 16 U.S.C. §§ 1531-1544. In the alternative, petitioners formally request that NMFS list the North American green sturgeon as a threatened species under the ESA. In either case, petitioners request that green sturgeon critical habitat be designated concurrent with the listing designation. This petition is filed under §553(e) of the Administrative Procedure Act (“APA” - 5 U.S.C. §§ 551-559), §1533(b)(3) of the ESA, and 50 C.F.R. §424.14(b). This petition sets in motion a specific administrative process as defined by §1533(b)(3) and 50 C.F.R. §424.14(b), placing mandatory response requirements on NMFS. Because A. medirostris is an anadromous fish, NMFS has jurisdiction over this petition. A Memorandum of Understanding (“MOU”) between NMFS and the U. -
Epa Contract No
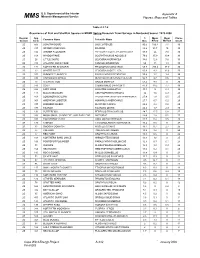
U.S. Department of the Interior Appendix A Minerals Management Service MM S Figures, Maps and Tables Table 4.2.7-6 Occurrence of Fish and Shellfish Species in MDMF Spring Research Trawl Surveys in Nantucket Sound: 1978-2008 Region/ Spp % Mean Mean Cruise Common Name Scientific Name Season Code Occ. #/Tow Wt/Tow Count 2S 503 LONGFIN SQUID LOLIGO PEALEII 90.5 100.4 7.1 30 2S 317 SPIDER CRAB UNCL MAJIDAE 88.2 93.7 10 30 2S 106 WINTER FLOUNDER PSEUDOPLEURONECTES AMERICANUS 87.9 26 7.8 30 2S 108 WINDOWPANE SCOPHTHALMUS AQUOSUS 79.6 35.9 10.4 30 2S 26 LITTLE SKATE LEUCORAJA ERINACEA 78.6 12.6 7.6 30 2S 313 ATLANTIC ROCK CRAB CANCER IRRORATUS 69 13 1.3 30 2S 171 NORTHERN SEAROBIN PRIONOTUS CAROLINUS 68.8 205.4 37.9 30 2S 23 WINTER SKATE LEUCORAJA OCELLATA 60.9 9.3 12.6 30 2S 103 SUMMER FLOUNDER PARALICHTHYS DENTATUS 55.4 1.7 1.4 30 2S 336 CHANNELED WHELK BUSYCOTYPUS CANALICULATUS 54.7 2.7 0.6 30 2S 73 ATLANTIC COD GADUS MORHUA 53.4 9.9 0 30 2S 143 SCUP STENOTOMUS CHRYSOPS 47.9 39.2 7.7 30 2S 322 LADY CRAB OVALIPES OCELLATUS 44.3 5 0.4 30 2S 141 BLACK SEA BASS CENTROPRISTIS STRIATA 30 1.6 0.9 29 2S 163 LONGHORN SCULPIN MYOXOCEPHALUS OCTODECEMSPINOSUS 27.9 1.8 0.5 25 2S 301 AMERICAN LOBSTER HOMARUS AMERICANUS 27.7 0.7 0.2 29 2S 337 KNOBBED WHELK BUSYCON CARICA 26.4 2.4 0.8 29 2S 177 TAUTOG TAUTOGA ONITIS 26.2 1.7 2.9 30 2S 131 BUTTERFISH PEPRILUS TRIACANTHUS 24.7 17.5 0.8 27 2S 338 MOON SNAIL, SHARK EYE, AND BABY-EAR NATICIDAE 24.4 1.8 0.1 27 2S 318 HORSESHOE CRAB LIMULUS POLYPHEMUS 20.7 0.4 0.5 28 2S 176 CUNNER TAUTOGOLABRUS ADSPERSUS 16.4 0.5 -

Monitoring Endangered Atlantic Sturgeon and Commercial Finfish Habitat Use in the New York Lease Area
OCS Study BOEM 2019-074 Monitoring Endangered Atlantic Sturgeon and Commercial Finfish Habitat Use in the New York Lease Area US Department of the Interior Bureau of Ocean Energy Management Office of Renewable Energy Programs OCS Study BOEM 2019-074 Monitoring Endangered Atlantic Sturgeon and Commercial Finfish Habitat Use in the New York Lease Area June 2019 Authors: Michael G. Frisk, Professor School of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook, New York 11794. Evan C. Ingram, Graduate Student School of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook, New York 11794. Keith Dunton, Assistant Professor Department of Biology, Monmouth University, 400 Cedar Avenue, West Long Branch, New Jersey 07764. Prepared under Cooperative Agreement M16AC00003 US Department of the Interior Bureau of Ocean Energy Management Office of Renewable Energy Programs DISCLAIMER Study collaboration and funding were provided by the US Department of the Interior, Bureau of Ocean Energy Management (BOEM), Environmental Studies Program, Washington, DC, under Agreement Number M16AC00003. This report has been technically reviewed by BOEM, and it has been approved for publication. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the US Government, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This project was funded by both BOEM and the New York State Department of Environmental Conservation (NYSDEC). REPORT AVAILABILITY To download a PDF file of this report, go to the US Department of the Interior, Bureau of Ocean Energy Management Data and Information Systems webpage (http://www.boem.gov/Environmental-Studies- EnvData/), click on the link for the Environmental Studies Program Information System (ESPIS), and search on 2019-074. -

PROGRESS REPORTS 2005 FISH DIVISION Oregon
PROGRESS REPORTS 2005 FISH DIVISION Oregon Department of Fish and Wildlife Final Summary Report: Green Sturgeon Population Characteristics in Oregon This program receives federal financial assistance in Sport Fish and/or Wildlife Restoration and prohibits discrimination on the basis of race, color, national origin, age, sex or disability. If you believe that you have been discriminated against as described above in any program, activity, or facility, or if you desire further information, please contact ADA Coordinator, Oregon Department of Fish and Wildlife, 3406 Cherry Avenue NE, Salem, OR, 97303, 503-947-6000, or write Office for Human Resources, U.S. Fish and Wildlife Service, Department of the Interior, Washington, D.C. 20240. This material will be furnished in alternate format for people with disabilities if needed. Please call (503) 657-2000 ext. 406 to request. FINAL PROGRESS REPORT FISH RESEARCH PROJECT OREGON PROJECT TITLE: Green Sturgeon Population Characteristics In Oregon PROJECT NUMBER: F-178-R JOB NUMBER: 1 JOB TITLE: Green Sturgeon Population Characteristics In Oregon PROJECT PERIOD: 1 October 1999 – 30 September 2004 Prepared by: Ruth A. Farr J. Chris Kern Oregon Department of Fish and Wildlife 17330 Southeast Evelyn Street Clackamas, OR 97015 This project was financed in part with the Federal Aid in Sport Fish Restoration Act (Wallop- Breaux) funds through the U. S. Fish and Wildlife Service. CONTENTS SUMMARY OF FINDINGS ............................................................................................... i INTRODUCTION