A Possible Increase in the Incidence of Congenital Heart Defects Among the Offspring of Affected Parents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How to Recognize a Suspected Cardiac Defect in the Neonate
Neonatal Nursing Education Brief: How to Recognize a Suspected Cardiac Defect in the Neonate https://www.seattlechildrens.org/healthcare- professionals/education/continuing-medical-nursing-education/neonatal- nursing-education-briefs/ Cardiac defects are commonly seen and are the leading cause of death in the neonate. Prompt suspicion and recognition of congenital heart defects can improve outcomes. An ECHO is not needed to make a diagnosis. Cardiac defects, congenital heart defects, NICU, cardiac assessment How to Recognize a Suspected Cardiac Defect in the Neonate Purpose and Goal: CNEP # 2092 • Understand the signs of congenital heart defects in the neonate. • Learn to recognize and detect heart defects in the neonate. None of the planners, faculty or content specialists has any conflict of interest or will be presenting any off-label product use. This presentation has no commercial support or sponsorship, nor is it co-sponsored. Requirements for successful completion: • Successfully complete the post-test • Complete the evaluation form Date • December 2018 – December 2020 Learning Objectives • Describe the risk factors for congenital heart defects. • Describe the clinical features of suspected heart defects. • Identify 2 approaches for recognizing congenital heart defects. Introduction • Congenital heart defects may be seen at birth • They are the most common congenital defect • They are the leading cause of neonatal death • Many neonates present with symptoms at birth • Some may present after discharge • Early recognition of CHD -

Congenital Cardiovascular Defects
Statistical Fact Sheet 2016 Update Congenital Cardiovascular Defects Congenital cardiovascular defects, also known as congenital heart defects, are structural problems that arise from abnormal formation of the heart or major blood vessels. ICD-9 lists 25 congenital heart defects codes, of which 21 designate specified anatomic or hemodynamic lesions. Defects range in severity from tiny pinholes between chambers that may resolve spontaneously to major malformations that can require multiple surgical procedures before school age and may result in death in utero, in infancy, or in childhood. The common complex defects include the following: Tetralogy of Fallot (TOF) Transposition of the great Trends in age-adjusted death rates attributable to arteries congenital heart defects, 1999 to 2013. Atrioventricular septal de- fects (ASD) Coarctation of the aorta Hypoplastic left heart syn- drome Incidence Congenital heart defects are serious and common condi- tions that have significant impact on morbidity, mortali- ty, and healthcare costs in children and adults. The most commonly report- ed incidence of congenital heart defects in the United States is between 4 and 10 per 1,000, clustering around 8 per 1,000 live births. Continental variations in birth prevalence have been reported, from 6.9 per 1000 births in Europe to 9.3 per 1000 in Asia. An estimated minimum of 40,000 infants are expected to be affected each year in the United States. Of these, about 25%, or 2.4 per 1,000 live births, require invasive treatment in the first year of life. Prevalence It is estimated that there are an estimated 650,000 to 1.3 million children and adults living with con- genital heart disease in the United States. -

Having an Echocardiogram to Screen for a Bicuspid Aortic Valve
Having an echocardiogram to screen for a bicuspid aortic valve UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm What is a bicuspid aortic valve? The aortic valve sits between the main chamber of the heart (the left ventricle) and the aorta. Its function is to ensure that blood flows correctly forward from the left ventricle into the aorta. Normally it has three thin leaflets which open as the heart contracts and then close to prevent back-flow of blood towards the ventricle. Aortic valve Tricuspid aortic valve (normal) Bicuspid aortic valve (abnormal) Some people may be born with an aortic valve made up of only 2 leaflets. The valve is then called bicuspid. This may cause problems with the functioning of the valve in that it may be more prone to gradually becoming narrowed or leaky. Sometimes a bicuspid valve may be associated with widening of the portion of the aorta that is connected to it. Widening of the aorta may occur in some relatives even if they have an aortic valve with 3 leaflets. If this widening is significant it is known as an aortic aneurysm. Is a bicuspid valve a common problem? Studies have suggested that this is not an uncommon valve problem and may occur in up to 1 in 200 people. 2 | PI18_1443_03 Having an echocardiogram to screen for a bicuspid aortic valve Recently there has been evidence that this condition may be genetic and thus have a tendency to run in a family. -

Echo in Asymptomatic Mitral and Aortic Regurgitation
2017 ASE Florida | Orlando, FL October 9, 2017 | 10:40 – 11:00 PM | 20 min | Grand Harbor Ballroom South Echo in Asymptomatic Mitral and Aortic Regurgitation Muhamed Sarić MD, PhD, MPA Director of Noninvasive Cardiology | Echo Lab Associate Professor of Medicine Disclosures Speakers Bureau (Philips, Medtronic) Advisory Board (Siemens) Regurgitation Axioms ▪Typically, regurgitation is NOT symptomatic unless severe ▪The opposite is not true: Severe regurgitation may be asymptomatic ▪ Chronic regurgitation leads to chamber dilatation on either side of the regurgitant valve Regurgitation Discovery ▪ Regurgitation as a anatomic entity was recognized in the 17th century ▪ Regurgitation was first clinically diagnosed by auscultation in the 19th century, well before the advent of echocardiography First Use of Regurgitation Term in English 1683 W. Charleton Three Anat. Lect. i. 18 Those [valves] that are placed in the inlet and outlet of the left Ventricle, to obviate the regurgitation of the bloud into the arteria venosa, and out of the aorta into the left Ventricle. Walter Charleton (1619 – 1707) English Physician Heart Murmur OXFORD ENGLISH DICTIONARY DEFINITION ▪ Any of various auscultatory sounds ▪ Adventitious sounds of cardiac or vascular origin [that is, separate from standard heart sounds: S1, S2, S3, S4] ▪ Sometimes of no significance ▪ But sometimes caused by valvular lesions of the heart or other diseases of the Στῆθος : Stēthos = chest circulatory system René Laënnec Stethoscope (1781 – 1826) (‘Chest examiner’) French Physician Hollow wooden cylinder Inventor of stethoscope in 1816 Laënnec Performing Auscultation Painted by Robert Alan Thom (1915 – 1979), American illustrator Commissioned by Parke, Davis & Co. 1816 1832 René Laënnec, James Hope French physician British physician Invents MONAURAL stethoscope separates MS from MR murmur 1852 1862 George Cammann Austin Flint Sr. -

Congenital Heart Disease Parent FAQ
Congenital Heart Disease Parent FAQ achd.stanfordchildrens.org | achd.stanfordhealthcare.org About Congenital Heart Disease What is congenital heart disease? Congenital heart disease, also called congenital heart defect (CHD), is a heart problem that a baby is born with. When the heart forms in the womb, it develops incorrectly and does not work properly, which changes how the blood flows through the heart. What causes congenital heart defects? In most cases, there is no clear cause. It can be linked to something out of the ordinary happening during gestation, including a viral infection or exposure to environmental factors. Or, it may be linked to a single gene defect or chromosome abnormalities. How common is CHD in the United States among children? Congenital heart defects are the most common birth defects in children in the United States. Approximately 1 in 100 babies are born with a heart defect. What are the most common types of congenital heart defects in children? In general, CHDs disrupt the flow of blood in the heart as it passes to the lungs or to the body. The most common congenital heart defects are abnormalities in the heart valves or a hole between the chambers of the heart (ventricles). Examples include ventricular septal defect (VSD), atrial septal defect (ASD), and bicuspid aortic valve. At the Betty Irene Moore Children’s Heart Center at Stanford Children’s Health, we are known across the nation and world for treating some of the most complex congenital heart defects with outstanding outcomes. Congenital Heart Disease Parent FAQ | 2 Is CHD preventable? In some cases, it could be preventable. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -

Congenital Mitral Incompetence and Coarctation Ofaorta
Thorax: first published as 10.1136/thx.27.6.729 on 1 November 1972. Downloaded from Thorax (1972), 27, 729. Congenital mitral incompetence and coarctation of aorta Report of two cases treated surgically' ABDEL K. TERZAKI2, ROBERT D. LEACHMAN3, M. KHALIL ALI, GRADY L. HALLMAN, and DENTON A. COOLEY Department of Medicine and the Cora and Webb Mading Department of Surgery, Baylor University College of Medicine, and the Cardiology and Surgical Sections of the Texas Heart Institute of St. Luke's Episcopal and Texas Children's Hospitals, Houston, Texas, U.S.A. Two patients with congenital mitral incompetence and coarctation of the aorta are presented. One patient had associated patent ductus arteriosus, bicuspid aortic valve, and endocardial fibro- elastosis. The diagnosis in the two patients presented is well established by clinical, laboratory, and surgical findings and also by necropsy examination in one case. It is proposed that the rarity of reported cases in the literature may have resulted from the frequent diagnosis of left ventricular failure in infancy secondary to coarctation, leading to the assumption that a mitral insufficiency murmur, when present, is due to functional regurgitation. Likewise, the murmur may be mistakenly thought to originate from a ventricular septal defect. The diagnosis of coarctation of the aorta presented no problem in either patient, while detection of the mitral incompetence was difficult. Coarctation of the aorta complicated by copyright. pulmonary hypertension in the absence of intracardiac shunt should draw attention to the possi- bility of associated mitral incompetence. Congestive heart failure, especially after correction of coarctation, was also an indication of possible associated mitral insufficiency. -

Prevalence of Migraine Headaches in Patients with Congenital Heart Disease
Prevalence of Migraine Headaches in Patients With Congenital Heart Disease Tam Truong, MD, Leo Slavin, MD, Ramin Kashani, BA, James Higgins, MD, Aarti Puri, BS, Malika Chowdhry, BS, Philip Cheung, BS, Adam Tanious, BA, John S. Child, MD, FAHA, Joseph K. Perloff, MD, and Jonathan M. Tobis, MD* The prevalence of migraine headaches (MH) is 12% in the general population and increases to 40% in patients with patent foramen ovale. This study evaluated the prevalence of MH in patients with congenital heart disease (CHD). Of 466 patients contacted from the UCLA Adult Congenital Heart Disease Center, 395 (85%) completed a questionnaire to determine the prevalence of MH. Patients were stratified by diagnosis of right-to-left, left-to-right, or no shunt. A group of 252 sex-matched patients with acquired cardiovascular disease served as controls. The prevalence of MH was 45% in adults with CHD compared to 11% in the controls (p <0.001). Of the 179 patients with MH, 143 (80%) had migraines with aura and 36 (20%) had migraines without aura versus 36% and 64% observed in the controls (p <0.001). The frequency of MH was 52% in the right-to-left shunt group, 44% in the left-to-right, and 38% in the no ,NS). In patients with a right-to-left shunt who underwent surgical repair ؍ shunt group (p 47% had complete resolution of MH, whereas 76% experienced >50% reduction in headache days per month. In conclusion, the prevalence of MH in all groups of adults with CHD is 3 to 4 times more than a sex-matched control population, with increasing prevalence of MH in patients with no shunt, left-to-right, and right-to-left shunt. -

Transcatheter Device Closure of Atrial Septal Defect in Dextrocardia with Situs Inversus Totalis
Case Report Nepalese Heart Journal 2019; Vol 16(1), 51-53 Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis Kiran Prasad Acharya1, Chandra Mani Adhikari1, Aarjan Khanal2, Sachin Dhungel1, Amrit Bogati1, Manish Shrestha3, Deewakar Sharma1 1 Department of Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal 2 Department of Internal Medicine, Kathmandu Medical College, Kathmandu,Nepal 3 Department of Pediatric Cardiology, Shahid Gangalal National Heart Centre, Kathmandu, Nepal Corresponding Author: Chandra Mani Adhikari Department of Cardiology Shahid Gangalal National Heart Centre Kathmandu, Nepal Email: [email protected] Cite this article as: Acharya K P, Adhikari C M, Khanal A, et al. Transcatheter device closure of atrial septal defect in dextrocardia with situs inversus totalis. Nepalese Heart Journal 2019; Vol 16(1), 51-53 Received date: 17th February 2019 Accepted date: 16th April 2019 Abstract Only few cases of Device closure of atrial septal defect in dextrocardia with situs inversus totalis has been reported previously. We present a case of a 36 years old male, who had secundum type of atrial septal defect in dextrocardia with situs inversus totalis. ASD device closure was successfully done. However, we encountered few technical difficulties in this case which are discussed in this case review. Keywords: atrial septal defect; dextrocardia; transcatheter device closure, DOI: https://doi.org/10.3126/njh.v16i1.23901 Introduction There are only two case reported of closure of secundum An atrial septal defect (ASD) is an opening in the atrial ASD associated in patients with dextrocardia and situs inversus septum, excluding a patent foramen ovale.1 ASD is a common totalis. -

Tetralogy of Fallot.” These Podcasts Are Designed to Give Medical Students an Overview of Key Topics in Pediatrics
PedsCases Podcast Scripts This is a text version of a podcast from Pedscases.com on “Tetralogy of Fallot.” These podcasts are designed to give medical students an overview of key topics in pediatrics. The audio versions are accessible on iTunes or at www.pedcases.com/podcasts. Tetralogy of Fallot Developed by Katie Girgulis, Dr. Andrew Mackie, and Dr. Karen Forbes for PedsCases.com. April 14, 2017 Introduction Hello, my name is Katie Girgulis and I am a medical student at the University of Alberta. This podcast was developed with the help of Dr. Andrew Mackie and Dr. Karen Forbes. Dr. Mackie is a pediatric cardiologist at the Stollery Children’s Hospital, and Dr. Forbes is a pediatrician and medical educator at the Stollery Children’s Hospital. This podcast is about the cardiac condition Tetralogy of Fallot (ToF). For teaching on the general approach to pediatric heart murmurs, please check out the ‘Evaluation of a Heart Murmur’ podcast on Pedscases.com. Slide 2 Learning Objectives By the end of this podcast, the learner will be able to: 1) Recognize the clinical presentations of ToF 2) Describe the four anatomical characteristics of ToF 3) Describe the pathophysiology of the murmur in ToF 4) Formulate initial steps when ToF is suspected 5) Delineate the treatment of hypercyanotic episodes 6) Summarize the definitive treatment for ToF Slide 3 Case – Baby Josh Let’s start with a clinical case: You are working with Dr. Smith, a family physician, during your family medicine rotation. Josh is a 4-month-old infant who is here for a well-baby check. -

Congenital Heart Disease: Recognition and Treatment Options – Anna Gelzer
CONGENITAL HEART DISEASE: RECOGNITION AND TREATMENT OPTIONS – ANNA GELZER Prevalence and Frequency Congenital heart disease in dogs: ≤ 1% (Canine clinic population of U Penn 1992) Cats estimated < 0.2% Prevalence likely underestimated (perinatal death, no murmur) Small % of cardiac disease overall, but most common in animals < 1 y old Common congenital cardiac defects in dogs (in the order of frequency): Subaortic stenosis (SAS) Pulmonic stenosis (PS) Patent ductus arteriosus (PDA) Tricuspid valve dysplasia (TVD) Ventricular septal defect (VSD) Tetralogy of Fallot (TOF) Persistent right aortic arch (PRAA) Atrial septal defects (ASD) Mitral valve dysplasia (MVD) Persistent left carnial venal cava Congenital cardiac defects in cats (in the order of frequency): Mitral valve dysplasia (MVD) Ventricular septal defect (VSD) Endocardial cushion defect (ASD+VSD) Patent ductus arteriosus (PDA) Aortic stenosis (SAS) Tetralogy of Fallot (TOF) Pulmonic stenosis (PS) Atrial septal defects (ASD) Tricuspid valve dysplasia (TVD) Physical exam finding in animals with most common congenital heart disease: The simple congenital heart defects are normally identified by the presence of a heart murmur. From the clinical examination, by localizing the heart murmur, identifying its radiation, assessing the precordial impulse and the peripheral pulse, a differential diagnosis list can be drawn up. The table summarizes the findings for some of the common defects identified in dogs and cats. Note, for these simple defects, mucus membrane color is normal (pink). -
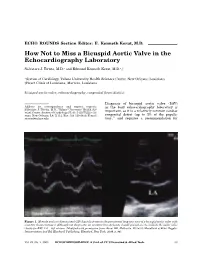
How Not to Miss a Bicuspid Aortic Valve in the Echocardiography Laboratory
ECHO ROUNDS Section Editor: E. Kenneth Kerut, M.D. How Not to Miss a Bicuspid Aortic Valve in the Echocardiography Laboratory Salvatore J. Tirrito, M.D.∗ and Edmund Kenneth Kerut, M.D.∗,† ∗Section of Cardiology, Tulane University Health Sciences Center, New Orleans, Louisiana †Heart Clinic of Louisiana, Marrero, Louisiana bicuspid aortic valve, echocardiography, congenital heart disease Diagnosis of bicuspid aortic valve (BAV) Address for correspondence and reprint requests: in the busy echocardiography laboratory is Salvatore J. Tirrito, M.D., Tulane University Health Sci- important, as it is a relatively common cardiac ences Center, Section of Cardiology SL-48, 1430 Tulane Av- enue, New Orleans, LA 71112. Fax: 504 529-9649; E-mail: congenital defect (up to 3% of the popula- 1 [email protected] tion), and requires a recommendation for Figure 1. M-mode and two-dimensional (2D) diastolic frame in the parasternal long-axis view of a bicuspid aortic valve with eccentric closure (arrows). Although not diagnostic, an eccentric line of closure should prompt one to evaluate the aortic valve closely for BAV. LA = left atrium. (Modified with permission from: Kerut EK, McIlwain, Plotnick: Handbook of Echo-Doppler Interpretation 2nd Ed, Blackwell Publishing, Elmsford, New York, 2004, p. 84) Vol. 22, No. 1, 2005 ECHOCARDIOGRAPHY: A Jrnl. of CV Ultrasound & Allied Tech. 53 TIRRITO AND KERUT Figure 2. Diastolic (left panel) and systolic (right panel) two-dimensional (2D) short-axis image of a BAV. In diastole the raphe may appear as a commissure (arrow), appearing to be a normal tri-leaflet valve. However,the systolic frame has a typical “fish-mouth” appearance.