Antimicrobial and in Vitro Enzyme Inhibitory Activities of Selected
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Asclepiadospermum Gen. Nov., the Earliest Fossil Record Of
RESEARCH ARTICLE Asclepiadospermum gen. nov., the earliest fossil record of Asclepiadoideae (Apocynaceae) from the early Eocene of central Qinghai-Tibetan Plateau, and its biogeographic implications Cédric Del Rio1,2, Teng-Xiang Wang1,3, Jia Liu1,2, Shui-Qing Liang1,4, Robert A. Spicer1,5, Fei-Xiang Wu6, Zhe-Kun Zhou1,2,7, and Tao Su1,2,3,8 Manuscript received 29 September 2019; revision accepted 18 PREMISE: Apocynaceae is common in the fossil record, especially as seed remains from the November 2019. Neogene of Europe and North America, but rare in Asia. Intrafamilial assignment is difficult 1 CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna due to the lack of diagnostic characters, and new fossil and modern data are needed to Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, understand the paleobiogeography of this group. Yunnan 666303, China 2 Center of Conservation Biology / Economic Botany / Plant METHODS: We studied three Apocynaceae seed impressions from the Lower Eocene Ecology, Core Botanical Gardens, Chinese Academy of Sciences, Niubao Formation, Jianglang village, Bangor County, central Qinghai-Tibetan Plateau. Mengla 666303, China Morphological data from living and fossil species were phylogenetically mapped to enable 3 University of Chinese Academy of Sciences, Beijing 100049, China systematic assignment. 4 Public Technology Service Center, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan RESULTS: We describe a new genus, Asclepiadospermum gen. nov., and two new species, 666303, China A. marginatum sp. nov. and A. ellipticum sp. nov. These species are characterized by an 5 School of Environment, Earth and Ecosystem Sciences, The Open elliptical seed, a margin surrounding the central part of the seed, and polygonal, irregular, University, Milton Keynes MK7 6AA, UK and small epidermal cells, and differ mainly in terms of the size of the margin and the 6 Key Laboratory of Vertebrate Evolution and Human shape of the apex. -
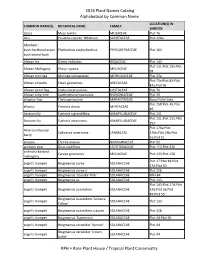
2016 Plant Names Catalog Alphabetical by Common Name
2016 Plant Names Catalog Alphabetical by Common Name LOCATION(S) IN COMMON NAME(S) BOTANICAL NAME FAMILY GARDEN abaca Musa textilis MUSACEAE Plot 76 abiu Pouteria caimito 'Whitman' SAPOTACEAE Plot 128a Abraham- bush:hardhead:scipio- Phyllanthus epiphyllanthus PHYLLANTHACEAE Plot 164 bush:sword-bush African iris Dietes iridioides IRIDACEAE Plot 143 Plot 131:Plot 19a:Plot African Mahogany Khaya nyasica MELIACEAE 58 African moringa Moringa stenopetala MORINGACEAE Plot 32a Plot 71a:Plot 83:Plot African oil palm Elaeis guineensis ARECACEAE 84a:Plot 96 African spiral flag Costus lucanusianus COSTACEAE Plot 76 African tulip-tree Spathodea campanulata BIGNONIACEAE Plot 29 alligator flag Thalia geniculata MARANTACEAE Royal Palm Lake Plot 158:Plot 45:Plot allspice Pimenta dioica MYRTACEAE 46 Amazon lily Eucharis x grandiflora AMARYLLIDACEAE Plot 131 Plot 131:Plot 151:Plot Amazon-lily Eucharis amazonica AMARYLLIDACEAE 152 Plot 176a:Plot American beauty Callicarpa americana LAMIACEAE 176b:Plot 19b:Plot berry 3a:Plot 51 anaqua Ehretia anacua BORAGINACEAE Plot 52 anchovy pear Grias cauliflora LECYTHIDACEAE Plot 112:Plot 32b andiroba:bastard Carapa guianensis MELIACEAE Plot 133:Plot 158 mahogany Plot 17:Plot 18:Plot angel's trumpet Brugmansia aurea SOLANACEAE 27d:Plot 50 angel's trumpet Brugmansia aurea x SOLANACEAE Plot 32b angel's trumpet Brugmansia 'Ecuador Pink' SOLANACEAE RPH-B4 angel's trumpet Brugmansia sp. SOLANACEAE Plot 133 Plot 143:Plot 27d:Plot angel's trumpet Brugmansia suaveolens SOLANACEAE 32b:Plot 3a:Plot 49:Plot 50 Brugmansia suaveolens -

Biologi Tanaman Industry, Ada Beberapa Pengertian
Diktat Kuliah BIOLOGI TANAMAN INDUS TRI Untuk Kalangan Mahasiswa Fakultas Biologi Universitas Medan Area Oleh: Drs. Riyanto, Msc Universitas Medan Area Medan 2012 UNIVERSITAS MEDAN AREA 2 KATA PENGANTAR Puji syukur dipanjatkan kehadirat Allah SWT atas selesainya penulisan Diktat Kuliah ini. Kami menyadari bahwa buku ini belum memuaskan para mahasiswa karena masih adanya kekurangan disana-sini. Untuk itu koreksi dan masukan dari rekan-rekan staff pengajar dan mahasiswa akan lebih menyempurnakannya untuk revisi dimasa datang. Semoga Diktat Kuliah ini dapat bermanfaat terutama bagi mahasiswa Fakultas Biologi UMA yang mengambil mata kuliah Biologi Tanaman lndustri. Medan, April 2012. Riyanto, Ors, Msc UNIVERSITAS MEDAN AREA 3 DAFTAR ISI Kuliah I: Pendahuluan Kuliah II: Biologi 'fanaman Serealia....... ................... Kuliah III: Biologi Tanaman Kacang-kacangan .................. ...... Kuliah IV: Biologi tanaman Umbi-umbian ............... .. ........ ... Kuliah V: Biologi Tanaman Hortikultura Buah-buahan ...... ..... Kuliah VI: Biologi Tanaman Hortikultura Sayur-sayuran .......... Kuliah VII: Biologi Tanaman Hortikultura Tanaman Hias ... ...... Kuliah VIII: Biologi Tanaman Hortikultura Tanaman Obat ............ Kuliah IX: Biologi Tanaman HTI ......................... ... .. Kuliah X: Biologi Tanaman Perkebunan Kelapa Sawit ............. .. Kuliah XI: Biologi Tanaman Perkebunan Karet .................... Kuliah XII: Biologi Tiuiaman Coldat, Kopi, Tea, Tembakau ........ Kuliah XIII: Biologi Tanaman Kapa'>, Nilam, Jarak ................ -

In Vitro Regeneration of an Endangered Ornamental Plant Impala Lily (Adenium Multiflorum Klotzsch)
Indian Journal of Fundamental and Applied Life Sciences ISSN: 2231-6345 (Online) An Online International Journal Available at http://www.cibtech.org/jls.htm 2012 Vol. 2 (3) July-September, pp.42-50/Tulika Talukdar Research Article IN VITRO REGENERATION OF AN ENDANGERED ORNAMENTAL PLANT IMPALA LILY (ADENIUM MULTIFLORUM KLOTZSCH) *Tulika Talukdar *Department of Botany, Krishnagar Govt. College, University of Kalyani, Krishnagar, 741101,West Bengal, India *Author for Correspondence ABSTRACT Adenium multiflorum Klotzsch is an endangered ornamental plant belonging to the family Apocynaceae. Poor seed germinability and very low multiplication rate of planting materials pose high threat to this pot plant, and its difficult propagation makes conservation essential. The present investigation is an effort to establish Adenium for micro-propagation and sub-culturing as a rapid, cost-effective alternative tool. Juvenile leaves were used as explants for in vitro organogenesis through callusing and cultured on three different basal media-MS, B-5 and N6 supplemented with phytohormones -α-naphthaleneacetic acid (NAA), kinetin and Gibberelic Acid (GA). Regenerated shoots were transferred to half strength basal media (MS, B-5 and N6) supplemented with 2% (w/v) sucrose, 0.8% difcobactoagar and different concentrations of growth regulators - NAA and kinetin for 1-2 weeks. The regenerated shoots were also tested on growth regulator free half strength basal media for rooting. The complete rooted plantlets were initially acclimatized in moist chamber and finally under semi-shady condition. This is the first report of complete regeneration of A. multiflorum from callus via shoot organogenesis. Key Words: Adenium Multiflorum Klotzsch, In Vitro, Ornamental, Regeneration, Basal Medium, PGRS INTRODUCTION Adenium multiflorum Klotzsch, also known as Impala Lily, is a beautiful ornamental plant in the family Apocynaceae. -

Ethnobotanical Survey in Canhane Village, District of Massingir
Ribeiro et al. Journal of Ethnobiology and Ethnomedicine 2010, 6:33 http://www.ethnobiomed.com/content/6/1/33 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access Ethnobotanical survey in Canhane village, district of Massingir, Mozambique: medicinal plants and traditional knowledge Ana Ribeiro1*, Maria M Romeiras1, João Tavares1, Maria T Faria2 Abstract Background: Medicinal plants are used by 80% of people from developing countries to fulfill their primary health needs, occupying a key position on plant research and medicine. Taking into account that, besides their pharmaceutical importance, these plants contribute greatly to ecosystems’ stability, a continuous documentation and preservation of traditional knowledge is a priority. The objective of this study was to organize a database of medicinal plants including their applications and associated procedures in Canhane village, district of Massingir, province of Gaza, Mozambique. Methods: In order to gather information about indigenous medicinal plants and to maximize the collection of local knowledge, eleven informants were selected taking into account the dimension of the site and the fact that the vegetation presents a great homogeneity. The data were collected through intensive structured and semi- structured interviews performed during field research. Taxonomical identification of plant species was based on field observations and herbarium collections. Results: A total of 53 plant species have been reported, which were used to treat 50 different human health problems. More than half of the species were used for stomach and intestine related disturbances (including major diseases such as diarrhea and dysentery). Additionally, four species with therapeutic applications were reported for the first time, whose potential can further be exploited. -

Australian Textile Exhibition 2017 28 February to 5 March 2017
VOLUME 23 — NUMBER 4 — SUMMER 2016 NEWSLETTER OF THE FRIENDS OF THE ROYAL BOTANIC GARDENS CRANBOURNE, INC. Australian Textile Our sixth AUSTRALIAN TEXTILE Exhibition 2017 EXHIBITION will be held in the Australian Garden Visitor Centre - both upstairs in the Gallery and downstairs in the Auditorium, 28 February to commencing on Tuesday February 28th and 5 March 2017 continuing through Sunday March 5th, from 10am to 4pm each day. Entry is FREE. The Exhibition will again be co-ordinated by Leesa Chandler of Chandlers Cottage and Highlights promises to offer another wonderful display of textile crafts, all inspired by our beautiful in this issue flora, fauna, landscapes and landmarks. The exhibition will also feature daily Microbats 7 demonstrations by leading textile artists in felting, quilting, embroidery and more, plus a Chandlers Cottage Pop Up shop, beautiful craftwork to buy and loads of inspiration for all. Leesa will also be displaying many “one- offs” of her own pieces, including bags, wall hangings and quilts. South Africa Tour Report 14 You will be able to purchase a wide range of patterns, kits and products from Chandlers This quilt titled ‘Whispering Gums’ is the first Cottage, plus items made by members of prize in the Cranbourne Friends Raffle at the the Friends of RBG Cranbourne ‘Botanical Textile Exhibition. Photo: Cass Merrigan. Fabricators’ group. by the Friends to assist the ongoing The 2017 Exhibition also sees the launch of development of the Cranbourne Gardens. It the Great Australian Bag Challenge which is Friends of the Royal Botanic is entitled ‘Whispering Gums‘. You can get a Gardens Cranbourne, Inc. -

Sennblad Et Al. 1998
American Journal of Botany 85(9): 1143±1158. 1998. MORPHOLOGY AND MOLECULAR DATA IN PHYLOGENETIC FRATERNITY: THE TRIBE WRIGHTIEAE (APOCYNACEAE) REVISITED1 BENGT SENNBLAD,2,4 MARY E. ENDRESS,3 AND BIRGITTA BREMER2 2 Department of Systematic Botany, Uppsala University, Villav. 6, S-752 36 Uppsala, Sweden; and 3 Institute of Systematic Botany, University of ZuÈrich, Zollikerstrasse 107, CH-8008 ZuÈrich, Switzerland The monophyly and classi®cation of the tribe Wrightieae of the subfamily Apocynoideae (Apocynaceae) are cladistically investigated. Nine taxa from the Wrightieae sensu Leeuwenberg, nine from other Apocynoideae sensu lato (s.l., including two from the traditional Asclepiadaceae), and two outgroup taxa from the Plumerioideae (Apocynaceae) were scored for rbcL sequence data and morphological data, mainly ¯oral characters, and analyzed using successive weighting parsimony analysis. The Wrightieae sensu Leeuwenberg are shown to be largely paraphyletic, the constituent taxa being dispersed among four monophyletic clades. Previously not suggested relationships indicated by the study are the association of Pachypodium with Funtumia, Holarrhena, and Mascarenhasia and the position of Beaumontia close to Trachelospermum. A reclassi®cation of the Wrightieae is discussed, in which three of the identi®ed clades are recognized as tribes, the Wrightieae sensu stricto (s.s.), the Nerieae, and the Malouetieae. The support for the Wrightieae s.s. is very strong, as evaluated with Bremer support and bootstrap analysis. The Malouetieae are also strongly supported, but the Nerieae less so. Using potential morphological synapomorphies identi®ed in the study, circumscription of the tribes is discussed. A potential pseudogene of rbcL is reported for Beaumontia. Key words: Apocynaceae; chloroplast DNA; classi®cation; ¯oral morphology; Malouetieae; Nerieae; phylogeny; rbcL; Wrightieae. -

Common Garden Plant List with Droplet Rating Customized to Harare’S Climate
Common Garden Plant List with droplet rating customized to Harare’s climate Droplet Rating: 1 = little to no water needed in addition to natural rainfall 2 = low to medium water requirements 3 = medium to high water requirements Popular indigenous trees for gardens Name Indigenous Droplet Wildlife Attractant Growth Habit Rating Acacia abyssinica (Nyanga flat-top) Zim 1 Birds/ Insects (pollen and protection) Large tree, umbrella shape Acacia sieberiana (paper bark) Zim 1 Birds/ Insects (pollen and protection) Large tree, umbrella shape Albizia glaberrima (loweveld Albizia) Zim 1 Birds/ Insects (pollen and protection) Large tree, umbrella shape, no thorns Bolusanthus specious (tree wisteria) Zim 1 Birds/ Insects (nectar) Small tree, flowers in spring Bridelia micrantha Zim 1 Birds/ Insects (nectar, fruit) Large tree, edible fruit Celtis africana (white stinkwood) Zim 1 Birds (esp. doves and seed eaters) Large tree, beautiful grey bark Cordia africana Zim 1 Birds (esp. louries and seed eaters) Large tree, beautiful white flowers Dais cotinifolia (pompon tree) Zim 1 Birds/ Insects (nectar) Medium tree, beautiful pink flowers Erythrina lysistemon (lucky bean) Zim 1 Birds/ Insects (nectar) Large tree, soft wood Fernandoa magnifica Zim 1 Birds/ Insects (nectar) Small tree, bright orange flowers Kiggelaria africana (wild peach) Zim 1 Birds (seeds) Small tree, striking seed pods Phoenix reclinata Zim 1 Birds (seeds) Multi-stem, tall palm tree Sclerocarya birrea (Marula) Zim 1 Birds (fruit) Medium tree, edible fruit Schotia brachypetala (weeping boerbean) Zim 1 Birds/ Insects (nectar) Small tree, red flowers Trema orientalis (pigeonwood) Zim 1 Birds (seeds) Small tree, very fast growing This document and the information contained therein is the intellectual property of Grow It Yourself (Pvt) Ltd. -

Desert Rose, Adenium Obesum Desert Rose, Mock Azalea, Impala Lily, and Sabi Star Are Amongst the Common Names of a Plant Available from a Mega- Store Near You
A Horticulture Information article from the Wisconsin Master Gardener website, posted 8 Feb 2013 Desert Rose, Adenium obesum Desert rose, mock azalea, impala lily, and Sabi star are amongst the common names of a plant available from a mega- store near you. Long grown by succulent plant enthusiasts because of its bizarre shape, beautiful fl owers in colors from deep red to pure white, and its tolerance of occasional neglect, adeniums are rapidly becoming popular horticultural subjects and houseplants worldwide. The Rose That Isn’t For one thing, it has no thorns. But beyond that, it is totally unrelated to the rose family and doesn’t really even look like one. So much for common names. The desert rose is An Adenium fl ower. scientifi cally known as Adenium obesum, or the fat adenium, referring to its grossly thickened trunk. It is in the Asclepiadaceae, or milkweed family, which, besides our garden milkweeds (Asclepias spp.) includes the common garden periwinkle, oleander (frequently used as fl oriferous landscape shrubs in mild climates such as Florida and southern California), the spiny Madagascar palm (which, or course, isn’t a palm at all), the fragrant frangipani, or Plumeria which is grown worldwide in tropical climates, and a myriad of African succulents with bizarre, often stinky, star-shaped fl owers, collectively referred to as stapeliads. Adenium is a small group of plants known from dry climates in sub- Saharan Africa and the very southern part of the Arabian Peninsula. Scientists debate how many wild species there are of Adenium. The most conservative view is that there is only one species and a handful of subspecies or varieties. -

05 Recommended Plant List
32.14. Recommended plant list Included in the architectural guidelines is a strictly indigenous tree planting policy: The landscaping and especially the trees, due to their size, go a long way to determining the over- all aesthetic ambience of an estate. With our indigenous landscaping policy we hope to reinforce the uniquely South African nature of this estate, not to mention the numerous additional advan- tages of planting indigenous trees as listed below: The bird life is significantly increased through the planting of indigenous trees that have berries for food, or thorns for protection from birds of prey. Frost resistance is another benefit of indigenous trees as they tend to be extremely hardy and are well adapted to our climate. Water is always a scarce commodity and indigenous trees require significantly less water than their exotic counterparts and have little or no effect on our fragile water table. They are also far more likely to survive a drought. Palms, Palm-like or large banana leaf-like plants are expressly forbidden as they create a tropical atmosphere, which is in conflict with the area which is essentially grassland and Bushveld. Plant material naturally includes trees, shrubs and ground cover species. TREES Acacia albida Acacia caffra Acacia galpinii Acacia karroo Albizia adianthifolia Bolusanthus speciosus Bridelia micrantha Buddleia aricyulata Buddleia saligna Buddleia salviifolia Celtis africana Chaetacme aristata Chrysophyllum viridifdium Combretum molle Combretum woodii Cussonia paniculata Eckebergia capensis Encephalartos -

2013 Board of Directors: Please See Our Website Calendar for the Next [email protected] Rescued Cactus Sale
TCSS BOARD Offi cers President: Richard Wiedhopf • [email protected] Vice President: Vonn Watkins • [email protected] Secretary: Susan Durham [email protected] Treasurer: Joe Frannea [email protected] Newsletter of the Tucson Cactus and Succulent Society January 2013 Board of Directors: Please see our Website Calendar for the next [email protected] rescued cactus sale. They are scheduled at various Thursday, January, 3, 2013 at 7:00 PM (Ending Dec. 31, 2013) times during the year based on our inventory. Linda Bartlett “The Road to Singapore” Cyndi Garrison TCSS Club Members receive a 10% discount Presented by Val Little with Mark Dimmitt and Gene Joseph William (Bill) Hicks Thomas Staudt January 2013 (Ending Dec. 31, 2014) Ed Bartlett Thursday, January 3, 2013 7:00pm Rob Romero Monthly Meeting, “The Road to Singapore” Joie Giunta presented by Val Little with Mark Dimmitt and Gene Joseph Bill Salisbury Tuesday, January 8, 2013 7:00pm (Ending Dec. 31, 2015) Board meeting at the U of A College of Pharmacy Keimpe Bronkhorst John Durham Linda Heisley Dale Johnson CSSA Affi liate Rep: Bill Holcombe (2013) Cactus Rescue [email protected] Aerial view of the Cloud Forest and Flower Dome, Gardens by the Bay, Cactus Rescue: Chris Monrad/Joe Frannea Singapore Education: Open Trained as both a Landscape Architect and an Anthropologist, Free Plants: Open Val Little is best known as the Director of the Water Conservation Floilegium: Margaret Pope • [email protected] Alliance of Southern Arizona. A long time traveller and plant Librarian: Joie Giunta • [email protected] geek, Val attended the opening of the new, Gardens by the Prickly Park: Jesse byrd (Implimentation) Bay, botanic garden in Singapore last summer. -

Download Plant List
Office Landline 035 550 4440 Will Hermon 078 019 1876 Thandeka Mguni 078 871 2247 email [email protected] MAYWOOD AVAILABILITY LIST Botanical Name Common Name Indigenous Tree 2L 4L 8L 15-20L 40-50L 100L Notes Indigenous Trees Botanical Name Common Name Indigenous Tree 2L 4L 8L 15-20L 40-50L 100L Notes Acacia / Senegalia Burkei Black Monkey Thorn Indigenous Tree R70.00 Acacia / Senegalia Galphini Monkey Thorn Indigenous R70.00 R900.00 Adansonia Digitata Baobab Tree Indigenous Tree R120.00 Afzelia Quanzensis Pod Mahogany Indigenous Tree R70.00 R220.00 Albizia Adianthifolia Flat Crown Falsethorn Indigenous Tree R70.00 Albizia Versicolor Large Leafed Falsethorn Indigenous Tree R70.00 Barringtonia Racemosa Powder Puff Tree Indigenous Tree R70.00 Bridelia Micrantha Mitzeeri Indigenous Tree R70.00 Buddleja Saligna False Olive Indigenous Tree R70.00 Burchellia bubalina Wild Pomegranate Indigenous Tree R70.00 Calodendrum Capense Wild Chestnut Indigenous Tree R70.00 Celtis Africana White Stinkwood Indigenous Tree R70.00 Clerodendron Glabrum Tinder Wood Indigenous Tree R70.00 Coddia Rudis Small Bone Apple, Kleinbeenappel Indigenous Tree R70.00 Combretum Erythrophyllum River Bush Willow Indigenous Tree R75.00 Cross Berry Grewia Occidentalis Indigenous Tree R75.00 Cussorina Spicata Cabbage Tree Indigenous Tree R75.00 Sago palm, king sago, sago cycad, Cycad Revoluta Indigenous Tree R70.00 R320.00 R650.00 Japanese sago palm Deinbollia oblongifolia Indigenous Tree R70.00 Dodonaea Viscosa Sand Olive Indigenous Tree R85.00 Dombeya Rotundifolia Wild