Sennblad Et Al. 1998
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolução Cromossômica Em Plantas De Inselbergues Com Ênfase Na Família Apocynaceae Juss. Angeline Maria Da Silva Santos
UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS AGRÁRIAS PÓS-GRADUAÇÃO EM AGRONOMIA CAMPUS II – AREIA-PB Evolução cromossômica em plantas de inselbergues com ênfase na família Apocynaceae Juss. Angeline Maria Da Silva Santos AREIA - PB AGOSTO 2017 UNIVERSIDADE FEDERAL DA PARAÍBA CENTRO DE CIÊNCIAS AGRÁRIAS PÓS-GRADUAÇÃO EM AGRONOMIA CAMPUS II – AREIA-PB Evolução cromossômica em plantas de inselbergues com ênfase na família Apocynaceae Juss. Angeline Maria Da Silva Santos Orientador: Prof. Dr. Leonardo Pessoa Felix Tese apresentada ao Programa de Pós-Graduação em Agronomia, Universidade Federal da Paraíba, Centro de Ciências Agrárias, Campus II Areia-PB, como parte integrante dos requisitos para obtenção do título de Doutor em Agronomia. AREIA - PB AGOSTO 2017 Catalogação na publicação Seção de Catalogação e Classificação S237e Santos, Angeline Maria da Silva. Evolução cromossômica em plantas de inselbergues com ênfase na família Apocynaceae Juss. / Angeline Maria da Silva Santos. - Areia, 2017. 137 f. : il. Orientação: Leonardo Pessoa Felix. Tese (Doutorado) - UFPB/CCA. 1. Afloramentos. 2. Angiospermas. 3. Citogenética. 4. CMA/DAPI. 5. Ploidia. I. Felix, Leonardo Pessoa. II. Título. UFPB/CCA-AREIA A Deus, pela presença em todos os momentos da minha vida, guiando-me a cada passo dado. À minha família Dedico esta conquista aos meus pais Maria Geovânia da Silva Santos e Antonio Belarmino dos Santos (In Memoriam), irmãos Aline Santos e Risomar Nascimento, tios Josimar e Evania Oliveira, primos Mayara Oliveira e Francisco Favaro, namorado José Lourivaldo pelo amor a mim concedido e por me proporcionarem paz na alma e felicidade na vida. Em especial à minha mãe e irmãos por terem me ensinado a descobrir o valor da disciplina, da persistência e da responsabilidade, indispensáveis para a construção e conquista do meu projeto de vida. -

A Revision of Farquharia Stapf and Funtumia Stapf (Apocynaceae)
582.937 MEDEDELINGEN LANDBOUWHOGESCHOOL WAGENINGEN • NEDERLAND •81-16(1981) A REVISION OF FARQUHARIA STAPF AND FUNTUMIA STAPF (APOCYNACEAE) H. J. C. ZWETSLOOT Laboratory of Plant Taxonomy and Plant Geography, Agricultural University, Wageningen, The Netherlands Received 21-VII-1981 Date of publication I1-XIM981 H. VEENMAN & ZONEN B.V. - WAGENINGEN - 1981 CONTENTS Introduction History Etymology Geographic distribution . Relationship to other genera Genus/species diagnosis of Farquharia 3 F. elliptica 3 Architecture of Farquharia 9 Nomina nuda referring to Farquharia 9 Phytochemical screening of Farquharia elliptica leaves by T. A. VAN BEEK 9 Genus diagnosis of Funtumia 10 Architecture of Funtumia 11 Discussion of the delimitation of the species of Funtumia 14 Key to the species of Funtumia 16 Species descriptions of Funtumia 16 F. africana 16 F. elastica 25 Somatic chromosome numbers in Funtumia by J. C. ARENDS and F. M. VAN DER LAAN . 32 Phytochemistry of Funtumia by N. G. BISSET 33 Hybrids of Funtumia africana and elastica 34 Statistical keys (Funtumia) 34 References 38 Acknowledgements 41 Index of exsiccatae 42 Register 46 INTRODUCTION The present publication is a monograph of the genera Farquharia and Fun- tumia. It is based on the study of herbarium material and living plants as the author had the opportunity to study flowering and fruiting plants in the field of all species involved. HISTORY Farquharia was described by STAPF in 1912 with a single species F. elliptica. Independantly CHEVALIER proposed the nomina nuda Alafiajasminiflora (1920) and Alqfia mirabilis for the same taxon. HUTCHINSON and DALZIEL (1931) er roneously referred Alafiajasminiflora to the genus Holalafia as they did not notice the clearly apocarpous ovary in the specimen collected by CHEVALIER. -

Check List of Wild Angiosperms of Bhagwan Mahavir (Molem
Check List 9(2): 186–207, 2013 © 2013 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution Check List of Wild Angiosperms of Bhagwan Mahavir PECIES S OF Mandar Nilkanth Datar 1* and P. Lakshminarasimhan 2 ISTS L (Molem) National Park, Goa, India *1 CorrespondingAgharkar Research author Institute, E-mail: G. [email protected] G. Agarkar Road, Pune - 411 004. Maharashtra, India. 2 Central National Herbarium, Botanical Survey of India, P. O. Botanic Garden, Howrah - 711 103. West Bengal, India. Abstract: Bhagwan Mahavir (Molem) National Park, the only National park in Goa, was evaluated for it’s diversity of Angiosperms. A total number of 721 wild species belonging to 119 families were documented from this protected area of which 126 are endemics. A checklist of these species is provided here. Introduction in the National Park are Laterite and Deccan trap Basalt Protected areas are most important in many ways for (Naik, 1995). Soil in most places of the National Park area conservation of biodiversity. Worldwide there are 102,102 is laterite of high and low level type formed by natural Protected Areas covering 18.8 million km2 metamorphosis and degradation of undulation rocks. network of 660 Protected Areas including 99 National Minerals like bauxite, iron and manganese are obtained Parks, 514 Wildlife Sanctuaries, 43 Conservation. India Reserves has a from these soils. The general climate of the area is tropical and 4 Community Reserves covering a total of 158,373 km2 with high percentage of humidity throughout the year. -

Apocynaceae-Apocynoideae)
THE NERIEAE (APOCYNACEAE-APOCYNOIDEAE) A. J. M. LEEUWENBERG1 ABSTRACT The genera of tribe Nerieae of Apocynaceae are surveyed here and the relationships of the tribe within the family are evaluated. Recent monographic work in the tribe enabled the author to update taxonomie approaches since Pichon (1950) made the last survey. Original observations on the pollen morphology ofth egener a by S.Nilsson ,Swedis h Natural History Museum, Stockholm, are appended to this paper. RÉSUMÉ L'auteur étudie lesgenre s de la tribu desNeriea e desApocynacée s et évalue lesrelation s del a tribu au sein de la famille. Un travail monographique récent sur la tribu a permit à l'auteur de mettre à jour lesapproche s taxonomiques depuis la dernière étude de Pichon (1950). Lesobservation s inédites par S. Nilsson du Muséum d'Histoire Naturelle Suédois à Stockholm sur la morphologie des pollens des genres sontjointe s à cet article. The Apocynaceae have long been divided into it to generic rank and in his arrangement includ two subfamilies, Plumerioideae and Apocynoi- ed Aganosma in the Echitinae. Further, because deae (Echitoideae). Pichon (1947) added a third, of its conspicuous resemblance to Beaumontia, the Cerberioideae, a segregate of Plumerioi it may well be that Amalocalyx (Echiteae— deae—a situation which I have provisionally ac Amalocalycinae, according to Pichon) ought to cepted. These subfamilies were in turn divided be moved to the Nerieae. into tribes and subtribes. Comparative studies Pichon's system is artificial, because he used have shown that the subdivision of the Plume the shape and the indumentum of the area where rioideae is much more natural than that of the the connectives cohere with the head of the pistil Apocynoideae. -

Floral Glands in Asclepiads: Structure, Diversity and Evolution
Acta Botanica Brasilica - 31(3): 477-502. July-September 2017. doi: 10.1590/0102-33062016abb0432 Review Floral glands in asclepiads: structure, diversity and evolution Diego Demarco1 Received: December 7, 2016 Accepted: February 24, 2017 . ABSTRACT Species of Apocynaceae stand out among angiosperms in having very complex fl owers, especially those of asclepiads, which belong to the most derived subfamily (Asclepiadoideae). Th ese fl owers are known to represent the highest degree of fl oral synorganization of the eudicots, and are comparable only to orchids. Th is morphological complexity may also be understood by observing their glands. Asclepiads have several protective and nuptial secretory structures. Th eir highly specifi c and specialized pollination systems are associated with the great diversity of glands found in their fl owers. Th is review gathers data regarding all types of fl oral glands described for asclepiads and adds three new types (glandular trichome, secretory idioblast and obturator), for a total of 13 types of glands. Some of the species reported here may have dozens of glands of up to 11 types on a single fl ower, corresponding to the largest diversity of glands recorded to date for a single structure. Keywords: anatomy, Apocynaceae, Asclepiadoideae, diversity, evolution, fl ower, secretory structures considering its most derived subfamily Asclepiadoideae. Introduction Th e close relationship between the former families Apocynaceae and Asclepiadaceae has always been recognized Apocynaceae is an extremely diverse family in since its establishment as “Apocineae” by Jussieu (1789). morphological terms, represented by trees, shrubs, herbs and climbers, with single leaves usually opposite, rarely Although Brown (1810) divided it into two families and alternate or whorled, with stipules modifi ed in colleters in this separation had been maintained in the subsequent several species (Endress & Bruyns 2000; Capelli et al. -

F^^R^-^-C."V.— Mededelingen Landbouwhogeschool Wageningen 82-4 (1982) (Communications Agricultural University) Isals O Published Asa Thesi S CONTENTS
581.961:582.937(6) MEDEDELINGEN LANDBOUWHOGESCHOOL WAGENINGEN• NEDERLAN D.82-4(1982 ) A MONOGRAPH ON STROPHANTHUS DC. (APOCYNACEAE) H.J. BEENTJE Department ofPlant Taxonomy andPlant Geography, Wageningen Agricultural University, The Netherlands Received19-V-198 2 Dateo fpublicatio n 15-10-82 H. VEENMAN&ZONENB.V.-WAGENINGEN-1982 -f^^r^-^-C."V.— Mededelingen Landbouwhogeschool Wageningen 82-4 (1982) (Communications Agricultural University) isals o published asa thesi s CONTENTS INTRODUCTION AND ACKNOWLEDGEMENTS 1 GENERAL PART: History of the genus 3 Geographical distribution and ecology 3 Habit and growth 6 Morphology 7 Flowering and fruiting seasons 7 Pollination 8 Dispersal of seeds 9 Anatomy 10 Chemistry and pharmacology 10 Palynology 11 Chromosome numbers, by J. C. ARENDS and F. M. VAN DER LAAN . 11 Local names 12 Uses and economic importance 12 Relationships with other genera 14 Citation of specimens 15 Definitions 15 TAXONOMIC PART: Genus diagnosis 17 Sectional arrangement 20 Discussion of the relationships within the genus 21 Key for flowering specimens 24 Key for specimens with leaves and mature fruits 31 Species diagnoses 35 Intermediates (possible hybrids) 164 Doubtful species 164 Nomina nuda 164 Excluded species 165 Old commercial names 166 List of names and synonyms not cited elsewhere in this revision ... 166 Index of exsiccatae 167 REFERENCES 183 REGISTER 189 INTRODUCTION AND ACKNOWLEDGEMENTS The present publication is a monograph on the genus Strophanthus, repre sented by 30 species in continental Africa, 1o n Madagascar, and 7 species in Asia. This monograph is based on the study of approximately 4700 herbarium specimens preserved in 54 herbaria. Living plants of 9 species were studied in the field and in cultivation. -

Ethnobotanical Knowledge of the Kuy and Khmer People in Prey Lang, Cambodia
Ethnobotanical knowledge of the Kuy and Khmer people in Prey Lang, Cambodia Turreira Garcia, Nerea; Argyriou, Dimitrios; Chhang, Phourin; Srisanga, Prachaya; Theilade, Ida Published in: Cambodian Journal of Natural History Publication date: 2017 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Turreira Garcia, N., Argyriou, D., Chhang, P., Srisanga, P., & Theilade, I. (2017). Ethnobotanical knowledge of the Kuy and Khmer people in Prey Lang, Cambodia. Cambodian Journal of Natural History, 2017(1), 76-101. http://www.fauna-flora.org/wp-content/uploads/CJNH-2017-June.pdf Download date: 26. Sep. 2021 76 N. Turreira-García et al. Ethnobotanical knowledge of the Kuy and Khmer people in Prey Lang, Cambodia Nerea TURREIRA-GARCIA1,*, Dimitrios ARGYRIOU1, CHHANG Phourin2, Prachaya SRISANGA3 & Ida THEILADE1,* 1 Department of Food and Resource Economics, University of Copenhagen, Rolighedsvej 25, 1958 Frederiksberg, Denmark. 2 Forest and Wildlife Research Institute, Forestry Administration, Hanoi Street 1019, Phum Rongchak, Sankat Phnom Penh Tmei, Khan Sen Sok, Phnom Penh, Cambodia. 3 Herbarium, Queen Sirikit Botanic Garden, P.O. Box 7, Maerim, Chiang Mai 50180, Thailand. * Corresponding authors. Email [email protected], [email protected] Paper submitted 30 September 2016, revised manuscript accepted 11 April 2017. ɊɮɍɅʂɋɑɳȶɆſ ȹɅƺɁɩɳȼˊɊNJȴɁɩȷ Ʌɩȶ ɑɒȴɊɅɿɴȼɍɈɫȶɴɇơȲɳɍˊɵƙɈɳȺˊƙɁȪɎLJɅɳȴȼɫȶǃNjɅȷɸɳɀɹȼɫȶɈɩɳɑɑ ɳɍˊɄɅDžɅɄɊƗƺɁɩɳǷȹɭɸ ɎȻɁɩ ɸɆɅɽɈɯȲɳȴɌɑɽɳǷʆ ɳDŽɹƺnjɻ ȶǁ ƳɌȳɮȷɆɌǒɩ Ə ɅLJɅɆɅƏɋȲƙɊɩɁɄɅDžɅɄɊƗƺɁɩɴȼɍDžƚ ɆɽNjɅ -

Asclepiadospermum Gen. Nov., the Earliest Fossil Record Of
RESEARCH ARTICLE Asclepiadospermum gen. nov., the earliest fossil record of Asclepiadoideae (Apocynaceae) from the early Eocene of central Qinghai-Tibetan Plateau, and its biogeographic implications Cédric Del Rio1,2, Teng-Xiang Wang1,3, Jia Liu1,2, Shui-Qing Liang1,4, Robert A. Spicer1,5, Fei-Xiang Wu6, Zhe-Kun Zhou1,2,7, and Tao Su1,2,3,8 Manuscript received 29 September 2019; revision accepted 18 PREMISE: Apocynaceae is common in the fossil record, especially as seed remains from the November 2019. Neogene of Europe and North America, but rare in Asia. Intrafamilial assignment is difficult 1 CAS Key Laboratory of Tropical Forest Ecology, Xishuangbanna due to the lack of diagnostic characters, and new fossil and modern data are needed to Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, understand the paleobiogeography of this group. Yunnan 666303, China 2 Center of Conservation Biology / Economic Botany / Plant METHODS: We studied three Apocynaceae seed impressions from the Lower Eocene Ecology, Core Botanical Gardens, Chinese Academy of Sciences, Niubao Formation, Jianglang village, Bangor County, central Qinghai-Tibetan Plateau. Mengla 666303, China Morphological data from living and fossil species were phylogenetically mapped to enable 3 University of Chinese Academy of Sciences, Beijing 100049, China systematic assignment. 4 Public Technology Service Center, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla, Yunnan RESULTS: We describe a new genus, Asclepiadospermum gen. nov., and two new species, 666303, China A. marginatum sp. nov. and A. ellipticum sp. nov. These species are characterized by an 5 School of Environment, Earth and Ecosystem Sciences, The Open elliptical seed, a margin surrounding the central part of the seed, and polygonal, irregular, University, Milton Keynes MK7 6AA, UK and small epidermal cells, and differ mainly in terms of the size of the margin and the 6 Key Laboratory of Vertebrate Evolution and Human shape of the apex. -

Introduction Medicinal Plants, Used by Both Ancient and Modern Cultures
Antioxidant Properties and Total Phenolic Content of Selected Traditional Thai Medicinal Plants นิพนธ์ต้นฉบ ับ Original Article พัชราภรณ์ ไชยศรี1* และ นงค์ลักษณ์ เหลาพรม2 Patcharaporn Chaisri1* and Nonglak Laoprom2 1 สาขาวิชาวิทยาศาสตร์สุขภาพ คณะวิทยาศาสตร์ มหาวิทยาลัยราชภัฏอุดรธานี จ.อุดรธานี 1 Department of Health Science, Faculty of Science, Udon Thani Rajabhat University, Udon 2 ภาควิชาวิทยาศาสตร์ทั่วไป คณะวิทยาศาสตร์และวิศวกรรมศาสตร์ มหาวิทยาลัยเกษตรศาสตร์ วิทยา Thani Province, Thailand เขตเฉลิมพระเกียรติ จ.สกลนคร 2 Department of General Science, Faculty of Science and Engineering, Kasetsart University Chalermphrakiat Sakon Nakhon Province Campus, Sakon Nakhon Province, Thailand * ติดต่อผู้นิพนธ์: [email protected] * Corresponding author: [email protected] วารสารไทยเภสชั ศาสตรแ์ ละวทิ ยาการสุขภาพ 2559;12(1):10-18. Thai Pharmaceutical and Health Science Journal 2016;12(1):10-18. บทค ัดย่อ Abstract วัตถุประสงค์: พืชสมุนไพรไทยตามภูมิปัญญาท้องถิ่นที่พบในภาค Objectives: In accordance with traditional local wisdom, medicinal plants ตะวันออกเฉียงเหนือถูกน ามาใช้รักษาบาดแผลทางผิวหนังและอักเสบ งานวิจัยนี้ from north-eastern Thailand are used for the treatment of dermatitis-related มวี ตั ถุประสงค์เพ่ือศึกษาฤทธติ์ ้านอนุมูลอิสระและปรมิ าณสารประกอบฟีนอลกิ inflammations. This study aimed to investigate the antioxidant activity and ทั้งหมด (total phenolic content; TPC) ของสารสกัดจากเปลือกพืชสมุนไพรไทย total phenolic content (TPC) of the bark of thirteen medicinal plants. ทั้งหมด 13 ชนิด วิธีการศึกษา: กระท่อมเลือด (Stephania venosa -

Apocynaceae) Author(S): David J
A Revision of Aganosma (Blume) G. Don (Apocynaceae) Author(s): David J. Middleton Source: Kew Bulletin, Vol. 51, No. 3 (1996), pp. 455-482 Published by: Springer on behalf of Royal Botanic Gardens, Kew Stable URL: http://www.jstor.org/stable/4117024 Accessed: 31/10/2010 14:53 Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at http://www.jstor.org/action/showPublisher?publisherCode=kew. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Royal Botanic Gardens, Kew and Springer are collaborating with JSTOR to digitize, preserve and extend access to Kew Bulletin. http://www.jstor.org A revision of Aganosma (Blume) G. Don (Apocynaceae) DAVID J. -
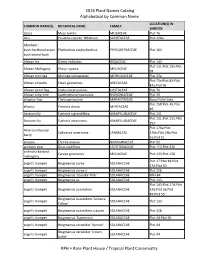
2016 Plant Names Catalog Alphabetical by Common Name
2016 Plant Names Catalog Alphabetical by Common Name LOCATION(S) IN COMMON NAME(S) BOTANICAL NAME FAMILY GARDEN abaca Musa textilis MUSACEAE Plot 76 abiu Pouteria caimito 'Whitman' SAPOTACEAE Plot 128a Abraham- bush:hardhead:scipio- Phyllanthus epiphyllanthus PHYLLANTHACEAE Plot 164 bush:sword-bush African iris Dietes iridioides IRIDACEAE Plot 143 Plot 131:Plot 19a:Plot African Mahogany Khaya nyasica MELIACEAE 58 African moringa Moringa stenopetala MORINGACEAE Plot 32a Plot 71a:Plot 83:Plot African oil palm Elaeis guineensis ARECACEAE 84a:Plot 96 African spiral flag Costus lucanusianus COSTACEAE Plot 76 African tulip-tree Spathodea campanulata BIGNONIACEAE Plot 29 alligator flag Thalia geniculata MARANTACEAE Royal Palm Lake Plot 158:Plot 45:Plot allspice Pimenta dioica MYRTACEAE 46 Amazon lily Eucharis x grandiflora AMARYLLIDACEAE Plot 131 Plot 131:Plot 151:Plot Amazon-lily Eucharis amazonica AMARYLLIDACEAE 152 Plot 176a:Plot American beauty Callicarpa americana LAMIACEAE 176b:Plot 19b:Plot berry 3a:Plot 51 anaqua Ehretia anacua BORAGINACEAE Plot 52 anchovy pear Grias cauliflora LECYTHIDACEAE Plot 112:Plot 32b andiroba:bastard Carapa guianensis MELIACEAE Plot 133:Plot 158 mahogany Plot 17:Plot 18:Plot angel's trumpet Brugmansia aurea SOLANACEAE 27d:Plot 50 angel's trumpet Brugmansia aurea x SOLANACEAE Plot 32b angel's trumpet Brugmansia 'Ecuador Pink' SOLANACEAE RPH-B4 angel's trumpet Brugmansia sp. SOLANACEAE Plot 133 Plot 143:Plot 27d:Plot angel's trumpet Brugmansia suaveolens SOLANACEAE 32b:Plot 3a:Plot 49:Plot 50 Brugmansia suaveolens -

Biologi Tanaman Industry, Ada Beberapa Pengertian
Diktat Kuliah BIOLOGI TANAMAN INDUS TRI Untuk Kalangan Mahasiswa Fakultas Biologi Universitas Medan Area Oleh: Drs. Riyanto, Msc Universitas Medan Area Medan 2012 UNIVERSITAS MEDAN AREA 2 KATA PENGANTAR Puji syukur dipanjatkan kehadirat Allah SWT atas selesainya penulisan Diktat Kuliah ini. Kami menyadari bahwa buku ini belum memuaskan para mahasiswa karena masih adanya kekurangan disana-sini. Untuk itu koreksi dan masukan dari rekan-rekan staff pengajar dan mahasiswa akan lebih menyempurnakannya untuk revisi dimasa datang. Semoga Diktat Kuliah ini dapat bermanfaat terutama bagi mahasiswa Fakultas Biologi UMA yang mengambil mata kuliah Biologi Tanaman lndustri. Medan, April 2012. Riyanto, Ors, Msc UNIVERSITAS MEDAN AREA 3 DAFTAR ISI Kuliah I: Pendahuluan Kuliah II: Biologi 'fanaman Serealia....... ................... Kuliah III: Biologi Tanaman Kacang-kacangan .................. ...... Kuliah IV: Biologi tanaman Umbi-umbian ............... .. ........ ... Kuliah V: Biologi Tanaman Hortikultura Buah-buahan ...... ..... Kuliah VI: Biologi Tanaman Hortikultura Sayur-sayuran .......... Kuliah VII: Biologi Tanaman Hortikultura Tanaman Hias ... ...... Kuliah VIII: Biologi Tanaman Hortikultura Tanaman Obat ............ Kuliah IX: Biologi Tanaman HTI ......................... ... .. Kuliah X: Biologi Tanaman Perkebunan Kelapa Sawit ............. .. Kuliah XI: Biologi Tanaman Perkebunan Karet .................... Kuliah XII: Biologi Tiuiaman Coldat, Kopi, Tea, Tembakau ........ Kuliah XIII: Biologi Tanaman Kapa'>, Nilam, Jarak ................