Issues of Random Sampling with Rapid Antigen Tests for COVID-19 Diagnosis: a Special Reference to Kalmunai RDHS Division
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Beautification and the Embodiment of Authenticity in Post-War Eastern Sri Lanka
University of Pennsylvania ScholarlyCommons Undergraduate Humanities Forum 2014-2015: Penn Humanities Forum Undergraduate Color Research Fellows 5-2015 Ornamenting Fingernails and Roads: Beautification and the Embodiment of Authenticity in Post-War Eastern Sri Lanka Kimberly Kolor University of Pennsylvania Follow this and additional works at: https://repository.upenn.edu/uhf_2015 Part of the Asian History Commons Kolor, Kimberly, "Ornamenting Fingernails and Roads: Beautification and the Embodiment of uthenticityA in Post-War Eastern Sri Lanka" (2015). Undergraduate Humanities Forum 2014-2015: Color. 7. https://repository.upenn.edu/uhf_2015/7 This paper was part of the 2014-2015 Penn Humanities Forum on Color. Find out more at http://www.phf.upenn.edu/annual-topics/color. This paper is posted at ScholarlyCommons. https://repository.upenn.edu/uhf_2015/7 For more information, please contact [email protected]. Ornamenting Fingernails and Roads: Beautification and the Embodiment of Authenticity in Post-War Eastern Sri Lanka Abstract In post-conflict Sri Lanka, communal tensions continue ot be negotiated, contested, and remade. Color codes virtually every aspect of daily life in salient local idioms. Scholars rarely focus on the lived visual semiotics of local, everyday exchanges from how women ornament their nails to how communities beautify their open—and sometimes contested—spaces. I draw on my ethnographic data from Eastern Sri Lanka and explore ‘color’ as negotiated through personal and public ornaments and notions of beauty with a material culture focus. I argue for a broad view of ‘public,’ which includes often marginalized and feminized public modalities. This view also explores how beauty and ornament are salient technologies of community and cultural authenticity that build on histories of ethnic imaginaries. -

CHAP 9 Sri Lanka
79o 00' 79o 30' 80o 00' 80o 30' 81o 00' 81o 30' 82o 00' Kankesanturai Point Pedro A I Karaitivu I. Jana D Peninsula N Kayts Jana SRI LANKA I Palk Strait National capital Ja na Elephant Pass Punkudutivu I. Lag Provincial capital oon Devipattinam Delft I. Town, village Palk Bay Kilinochchi Provincial boundary - Puthukkudiyiruppu Nanthi Kadal Main road Rameswaram Iranaitivu Is. Mullaittivu Secondary road Pamban I. Ferry Vellankulam Dhanushkodi Talaimannar Manjulam Nayaru Lagoon Railroad A da m' Airport s Bridge NORTHERN Nedunkeni 9o 00' Kokkilai Lagoon Mannar I. Mannar Puliyankulam Pulmoddai Madhu Road Bay of Bengal Gulf of Mannar Silavatturai Vavuniya Nilaveli Pankulam Kebitigollewa Trincomalee Horuwupotana r Bay Medawachchiya diya A d o o o 8 30' ru 8 30' v K i A Karaitivu I. ru Hamillewa n a Mutur Y Pomparippu Anuradhapura Kantalai n o NORTH CENTRAL Kalpitiya o g Maragahewa a Kathiraveli L Kal m a Oy a a l a t t Puttalam Kekirawa Habarane u 8o 00' P Galgamuwa 8o 00' NORTH Polonnaruwa Dambula Valachchenai Anamaduwa a y O Mundal Maho a Chenkaladi Lake r u WESTERN d Batticaloa Naula a M uru ed D Ganewatta a EASTERN g n Madura Oya a G Reservoir Chilaw i l Maha Oya o Kurunegala e o 7 30' w 7 30' Matale a Paddiruppu h Kuliyapitiya a CENTRAL M Kehelula Kalmunai Pannala Kandy Mahiyangana Uhana Randenigale ya Amparai a O a Mah Reservoir y Negombo Kegalla O Gal Tirrukkovil Negombo Victoria Falls Reservoir Bibile Senanayake Lagoon Gampaha Samudra Ja-Ela o a Nuwara Badulla o 7 00' ng 7 00' Kelan a Avissawella Eliya Colombo i G Sri Jayewardenepura -

Sri Lanka – Tamils – Eastern Province – Batticaloa – Colombo
Refugee Review Tribunal AUSTRALIA RRT RESEARCH RESPONSE Research Response Number: LKA34481 Country: Sri Lanka Date: 11 March 2009 Keywords: Sri Lanka – Tamils – Eastern Province – Batticaloa – Colombo – International Business Systems Institute – Education system – Sri Lankan Army-Liberation Tigers of Tamil Eelam conflict – Risk of arrest This response was prepared by the Research & Information Services Section of the Refugee Review Tribunal (RRT) after researching publicly accessible information currently available to the RRT within time constraints. This response is not, and does not purport to be, conclusive as to the merit of any particular claim to refugee status or asylum. This research response may not, under any circumstance, be cited in a decision or any other document. Anyone wishing to use this information may only cite the primary source material contained herein. Questions 1. Please provide information on the International Business Systems Institute in Kaluvanchikkudy. 2. Is it likely that someone would attain a high school or higher education qualification in Sri Lanka without learning a language other than Tamil? 3. Please provide an overview/timeline of relevant events in the Eastern Province of Sri Lanka from 1986 to 2004, with particular reference to the Sri Lankan Army (SLA)-Liberation Tigers of Tamil Eelam (LTTE) conflict. 4. What is the current situation and risk of arrest for male Tamils in Batticaloa and Colombo? RESPONSE 1. Please provide information on the International Business Systems Institute in Kaluvanchikkudy. Note: Kaluvanchikkudy is also transliterated as Kaluwanchikudy is some sources. No references could be located to the International Business Systems Institute in Kaluvanchikkudy. The Education Guide Sri Lanka website maintains a list of the “Training Institutes Registered under the Ministry of Skills Development, Vocational and Tertiary Education”, and among these is ‘International Business System Overseas (Pvt) Ltd’ (IBS). -

Update UNHCR/CDR Background Paper on Sri Lanka
NATIONS UNIES UNITED NATIONS HAUT COMMISSARIAT HIGH COMMISSIONER POUR LES REFUGIES FOR REFUGEES BACKGROUND PAPER ON REFUGEES AND ASYLUM SEEKERS FROM Sri Lanka UNHCR CENTRE FOR DOCUMENTATION AND RESEARCH GENEVA, JUNE 2001 THIS INFORMATION PAPER WAS PREPARED IN THE COUNTRY RESEARCH AND ANALYSIS UNIT OF UNHCR’S CENTRE FOR DOCUMENTATION AND RESEARCH ON THE BASIS OF PUBLICLY AVAILABLE INFORMATION, ANALYSIS AND COMMENT, IN COLLABORATION WITH THE UNHCR STATISTICAL UNIT. ALL SOURCES ARE CITED. THIS PAPER IS NOT, AND DOES NOT, PURPORT TO BE, FULLY EXHAUSTIVE WITH REGARD TO CONDITIONS IN THE COUNTRY SURVEYED, OR CONCLUSIVE AS TO THE MERITS OF ANY PARTICULAR CLAIM TO REFUGEE STATUS OR ASYLUM. ISSN 1020-8410 Table of Contents LIST OF ACRONYMS.............................................................................................................................. 3 1 INTRODUCTION........................................................................................................................... 4 2 MAJOR POLITICAL DEVELOPMENTS IN SRI LANKA SINCE MARCH 1999................ 7 3 LEGAL CONTEXT...................................................................................................................... 17 3.1 International Legal Context ................................................................................................. 17 3.2 National Legal Context........................................................................................................ 19 4 REVIEW OF THE HUMAN RIGHTS SITUATION............................................................... -

Divisional Secretariats Contact Details
Divisional Secretariats Contact Details District Divisional Secretariat Divisional Secretary Assistant Divisional Secretary Life Location Telephone Mobile Code Name E-mail Address Telephone Fax Name Telephone Mobile Number Name Number 5-2 Ampara Ampara Addalaichenai [email protected] Addalaichenai 0672277336 0672279213 J Liyakath Ali 0672055336 0778512717 0672277452 Mr.MAC.Ahamed Naseel 0779805066 Ampara Ampara [email protected] Divisional Secretariat, Dammarathana Road,Indrasarapura,Ampara 0632223435 0632223004 Mr.H.S.N. De Z.Siriwardana 0632223495 0718010121 063-2222351 Vacant Vacant Ampara Sammanthurai [email protected] Sammanthurai 0672260236 0672261124 Mr. S.L.M. Hanifa 0672260236 0716829843 0672260293 Mr.MM.Aseek 0777123453 Ampara Kalmunai (South) [email protected] Divisional Secretariat, Kalmunai 0672229236 0672229380 Mr.M.M.Nazeer 0672229236 0772710361 0672224430 Vacant - Ampara Padiyathalawa [email protected] Divisional Secretariat Padiyathalawa 0632246035 0632246190 R.M.N.Wijayathunga 0632246045 0718480734 0632050856 W.Wimansa Senewirathna 0712508960 Ampara Sainthamarathu [email protected] Main Street Sainthamaruthu 0672221890 0672221890 Mr. I.M.Rikas 0752800852 0672056490 I.M Rikas 0777994493 Ampara Dehiattakandiya [email protected] Divisional Secretariat, Dehiattakandiya. 027-2250167 027-2250197 Mr.R.M.N.C.Hemakumara 027-2250177 0701287125 027-2250081 Mr.S.Partheepan 0714314324 Ampara Navithanvelly [email protected] Divisional secretariat, Navithanveli, Amparai 0672224580 0672223256 MR S.RANGANATHAN 0672223256 0776701027 0672056885 MR N.NAVANEETHARAJAH 0777065410 0718430744/0 Ampara Akkaraipattu [email protected] Main Street, Divisional Secretariat- Akkaraipattu 067 22 77 380 067 22 800 41 M.S.Mohmaed Razzan 067 2277236 765527050 - Mrs. A.K. Roshin Thaj 774659595 Ampara Ninthavur Nintavur Main Street, Nintavur 0672250036 0672250036 Mr. T.M.M. -

Name List of Sworn Translators in Sri Lanka
MINISTRY OF JUSTICE Sworn Translator Appointments Details 1/29/2021 Year / Month Full Name Address NIC NO District Court Tel No Languages November Rasheed.H.M. 76,1st Cross Jaffna Sinhala - Tamil Street,Ninthavur 12 Sinhala - English Sivagnanasundaram.S. 109,4/2,Collage Colombo Sinhala - Tamil Street,Kotahena,Colombo 13 Sinhala - English Dreyton senaratna 45,Old kalmunai Baticaloa Sinhala - Tamil Road,Kalladi,Batticaloa Sinhala - English 1977 November P.M. Thilakarathne Chilaw 0777892610 Sinhala - English P.M. Thilakarathne kirimathiyana East, Chilaw English - Sinhala Lunuwilla. S.D. Cyril Sadanayake 26, De silva Road, 331490350V Kalutara 0771926906 English - Sinhala Atabagoda, Panadura 1979 July D.A. vincent Colombo 0776738956 English - Sinhala 1 1/29/2021 Year / Month Full Name Address NIC NO District Court Tel No Languages 1992 July H.M.D.A. Herath 28, Kolawatta, veyangda 391842205V Gampaha 0332233032 Sinhala - English 2000 June W.A. Somaratna 12, sanasa Square, Gampaha 0332224351 English - Sinhala Gampaha 2004 July kalaichelvi Niranjan 465/1/2, Havelock Road, Colombo English - Tamil Colombo 06 2008 May saroja indrani weeratunga 1E9 ,Jayawardanagama, colombo English - battaramulla Sinhala - 2008 September Saroja Indrani Weeratunga 1/E/9, Jayawadanagama, Colombo Sinhala - English Battaramulla 2011 July P. Maheswaran 41/B, Ammankovil Road, Kalmunai English - Sinhala Kalmunai -2 Tamil - K.O. Nanda Karunanayake 65/2, Church Road, Gampaha 0718433122 Sinhala - English Gampaha 2011 November J.D. Gunarathna "Shantha", Kalutara 0771887585 Sinhala - English Kandawatta,Mulatiyana, Agalawatta. 2 1/29/2021 Year / Month Full Name Address NIC NO District Court Tel No Languages 2012 January B.P. Eranga Nadeshani Maheshika 35, Sri madhananda 855162954V Panadura 0773188790 English - French Mawatha, Panadura 0773188790 Sinhala - 2013 Khan.C.M.S. -

Tides of Violence: Mapping the Sri Lankan Conflict from 1983 to 2009 About the Public Interest Advocacy Centre
Tides of violence: mapping the Sri Lankan conflict from 1983 to 2009 About the Public Interest Advocacy Centre The Public Interest Advocacy Centre (PIAC) is an independent, non-profit legal centre based in Sydney. Established in 1982, PIAC tackles barriers to justice and fairness experienced by people who are vulnerable or facing disadvantage. We ensure basic rights are enjoyed across the community through legal assistance and strategic litigation, public policy development, communication and training. 2nd edition May 2019 Contact: Public Interest Advocacy Centre Level 5, 175 Liverpool St Sydney NSW 2000 Website: www.piac.asn.au Public Interest Advocacy Centre @PIACnews The Public Interest Advocacy Centre office is located on the land of the Gadigal of the Eora Nation. TIDES OF VIOLENCE: MAPPING THE SRI LANKAN CONFLICT FROM 1983 TO 2009 03 EXECUTIVE SUMMARY ....................................................................................................................... 09 Background to CMAP .............................................................................................................................................09 Report overview .......................................................................................................................................................09 Key violation patterns in each time period ......................................................................................................09 24 July 1983 – 28 July 1987 .................................................................................................................................10 -
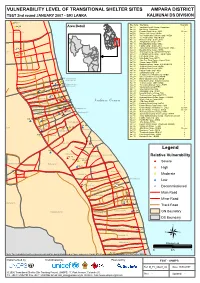
VULNERABILITY LEVEL of TRANSITIONAL SHELTER SITES AMPARA DISTRICT TSST 2Nd Round JANUARY 2007 - SRI LANKA KALMUNAI DS DIVISION
VULNERABILITY LEVEL OF TRANSITIONAL SHELTER SITES AMPARA DISTRICT TSST 2nd round JANUARY 2007 - SRI LANKA KALMUNAI DS DIVISION *#Kal_16 Site Code Site Name Total Vul. Kal_02 *# Area Detail Kal_01 Varathan RiceMill Camp - Samaritans 1 Kal_02 Bar Camp - Samaritan 1 Periyaneelavanai 01B Kal_04 Ponniah Road Camp - WDC Decom Kal_06 Vishnu Kovil Camp - NHDA 1 Kal_07 VC Road Muslim Division Camp - NHDA 1 Kal_08 V.C.Road Camp - WDC/NHDA 1 Kal_12 Moossa Camp - Sewa Lanka 2 Kal_15 Sanganthurai Camp - JVP 1 Kal_16 Masjidul Aqbar Camp - WDC 1 Kal_17 Hudha Camp - Sewa Lanka 1 Kal_04 Kal_18 Aqbar Jummah Mosque Road Camp - WDC 3 0* Kal_19 Masjidul Aqbar Camp - NHDA 2 ai 01A Kal_20 S.M.Road Camp - UNHCR/EHED/FCE 2 *#Kal_01 Kal_21 Al-Manar Central Camp - FORUT/WDC 1 Kal_22 Hijra Road Camp - JVP 2 Kal_23 Hijra Road Camp -MFCD 2 Kal_24 Sam Sum Road Camp - Islamic Relief 1 Kal_25 Shums Camp - EHED 2 Kal_26 Maqbooliya Lane Camp - EHED/UNHCR 2 Kal_27 Al-Minah Road Camp - NHDA 1 Kal_28 Al-Minan Road Camp - WDC 1 Kal_30 Hajiyar Road Camp - WDC 1 Kal_31 Sahanthura Camp - JVP 2 Kal_33 Sellappa Cross Road Camp - EHED 1 Kal_34 Valluvar(AI-Minan Camp)-EHED 2 Kal_17 Kal_12 Periyaneelavanai Kal_35 Maha Vidyalaya Camp - EHED 2 *# Muslim Sec.02 Kal_07 *# Kal_37 Khali Kovil Camp - NHDA/Sevalanka 2 *# *# *# Kal_38 Thathan Camp - MSF / EHED 1 Kal_06 Periyaneelavanai Kal_39 Thiroufathy Amman Camp - EHED 1 Kal_08 Muslim Sec.01 Kal_68 Kal_40 Vipulananda Camp-EHED 1 Periyaneelavanai 02 Kal_69 Kal_20 Kal_41 Visuvasikal Illam Camp 1 *# Kal_15 *# Kal_19 *# Kal_27*# *# *# Kal_43 -

IASC Inter-Agency Standing Committee Country Team
Inter-Agency Standing Committee Country Team IASC Colombo Sri Lanka SITUATION REPORT # 143 JAFFNA, KILINOCHCHI, MULLAITIVU, MANNAR, VAVUNIYA, TRINCOMALEE, BATTICALOA and AMPARA DISTRICTS 4-11 September 2008 IDP situation as reported this week by GAs Displacement after April 2006 Location Number of Families Number of Individuals Jaffna 6,129 20,736 Kilinochchi 35,331 136,190 Mullaitivu 8,917 31,080 Vavuniya 5,205 18,429 Mannar 2,754 8,623 Trincomalee 1,480 4,896 Batticaloa 4,111 15,612 Ampara 1,162 4,278 JAFFNA DISTRICT Situation update • The general security situation in the Jaffna peninsula remained tense during the week. • There is a tense situation amongst humanitarian agencies about the situation in the Vanni. Displacement/Returns • No changes reported. Sector Developments/Gaps • Following a court decision on returns to the Tellipalai HSZ, IDPs who wish to return to their places of origin within area are being registered. There seems to be a possibility that a section of the buffer zone south of the HSZ in Tellipalai will be released. However, it will be a lengthy process since mine clearance activities have not yet commenced. • In light of the development in the Vanni, the sector leads and sector working groups have started to review the contingency plans which were last updated 6 months ago. A review of the emergency stocks is also underway. KILINOCHCHI & MULLAITHIVU DISTRICTS Situation update • On September 12, a few hundred protesters, mostly IDPs, gathered in front of both the UNHCR and WFP compounds expressing concerns about the Un agencies leaving the Vanni. Negotiations with the protesters failed to enable the departure of a small convoy of INGO/UN vehicles that were scheduled to leave. -
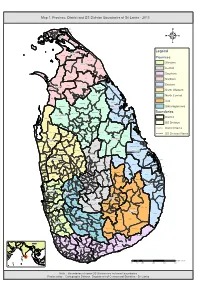
Map 1: Province, District and DS Division Boundaries of Sri Lanka - 2013
Map 1: Province, District and DS Division Boundaries of Sri Lanka - 2013 Vadam aradchi North (P oint Pedro) Valikam am North (Tellipallai) Valikam am S outh- West ( Sandilipay ) Vadam aradchi South-W es t (K araveddy) Valikam am W est (Chankanai) Karainagar Valikam am E ast (K opay) Valikam am S outh ( Uduv il) Jaffna Is land North (K ayts ) Thenm aradchi (Chavak achcheri) Jaffna Nallur Is land S outh (V elanai) Vadam aradchi Eas t Pachc hilaipalli 4 Delft Kandavalai Legend Kilinochchi Poonakary Karac hchi Provinces Puthukk udiyiruppu Mullaitivu Western Thunuk kai Maritim epattu Oddus uddan Central Mannar Town Southern Manthai W es t Manthai E ast Vav uniy a Nor th Welioya Northern Padavi Sr i P ura Madhu Eastern Mannar Vavuniya Padaviya Nanattan Vav uniy a Kuchc haveli North Western Vav uniy a S outh Gom ar ank adawala Kebithigollewa North Central Mus ali Vengalacheddik ulam Uva Morawewa Medawachc hiya Tr inc omalee Town and Gr av ets Mahawilachc hiy a Trincomalee Hor owpothana Sabaragamuwa Tham balagam uwa Ram bewa Kahatagas digiliya Kinniya Muttur Nuwar agam Palatha Central Boundaries Anuradhapura Mihinthale Kanthale Vanathawilluwa Noc hchiyagama District Nuwar agam Palatha E ast Seruv ila Verugal (E achc hilam pattu) Galenbindunuwewa Nac hchaduwa DS Division Thirappane Thalawa Medirigiriya Rajanganay a Colombo Tham buttegam a District Name Karuwalagas wewa Gir ibawa Hingurakgoda Ipalogam a Palugas wewa Kolonnawa Lank apura DS Division Name Welikanda Koralai P attu North (V aharai) Puttalam Galnewa Nawagattegam a Galgam uwa Kekirawa -

5000-Schools-Funded-By-The-Ministry
5000 Schools developed as Child Frendly Schools by funding Rs 500,000.00 by Economic Development Ministry to develop infastructure Province District Name of School Address Education Zone Education Division 1 Western Colombo SRI SANGAMITTA P.V. 62,ANANDA RAJAKARUNA MW.,COL-09 Colombo Borella 2 Western Colombo SUJATHA B.V. KIRIMANDALA MW.,COL-05 Colombo Colombo - South 3 Western Colombo LUMBINI P.V. HAVELOCK TOWN,COL-05. Colombo Colombo - South 4 Western Colombo ST.CLARE'S B.M.V. 1SR CHAPEL LANE,COL-06. Colombo Colombo - South 5 Western Colombo THANNINAYAGAM T.V. LESLEY RANAGALA MW.,COL-08 Colombo Borella 6 Western Colombo SIR BARON JAYATHILAKA V. MALIGAWATTA,COL-10. Colombo Colombo - Central 7 Western Colombo MIHINDU MAWATHA SINHALA V. MIHINDU MAWATHA,COLOMBO 12. Colombo Colombo - Central 8 Western Colombo ROMAN CATHOLIC V. KOTIKAWATTA, MULLERIYAWA NEW TOWN. Sri Jaya' pura Kolonnawa 9 Western Colombo MEETHOTAMULLA SRI RAHULA V. MEETHOTAMULLA, KOLONNAWA. Sri Jaya' pura Kolonnawa 10 Western Colombo KOTUWILA GAMINI V. KOTUWILA, WELLAMPITIYA. Sri Jaya' pura Kolonnawa 11 Western Colombo WERAGODA K.V. KOLONNAWA, WELLAMPITIYA. Sri Jaya' pura Kolonnawa 12 Western Colombo GOTHATUWA M.V. GOTHATUWA, ANGODA. Sri Jaya' pura Kolonnawa 13 Western Colombo VIDYAWARDENA V. WELLAMPITIYA, KOLONNAWA. Sri Jaya' pura Kolonnawa 14 Western Colombo SUGATHADHARMADHARA V. EGODAUYANA, MORATUWA Piliyandala Moratuwa 15 Western Colombo KATUKURUNDA ST MARY'S V. KATUKURUNDA, MORATUWA Piliyandala Moratuwa 16 Western Colombo SRI SADDARMODAYA V. KORALAWELLA MORATUWA Piliyandala Moratuwa 17 Western Colombo SRI NAGASENA V. KORAWELLA, MORATUWA Piliyandala Moratuwa 18 Western Colombo PITIPANA K.V. PITIPANA NORTH, HOMAGAMA. Homagama Homagama 19 Western Colombo DOLAHENA K.V. -

Humanitarian Operation Factual Analysis July 2006 – May 2009
HUMANITARIAN OPERATION FACTUAL ANALYSIS JULY 2006 – MAY 2009 MINISTRY OF DEFENCE DEMOCRATIC SOCIALIST REPUBLIC OF SRI LANKA HUMANITARIAN OPERATION FACTUAL ANALYSIS JULY 2006 – MAY 2009 MINISTRY OF DEFENCE JULY 2011 DEMOCRATIC SOCIALIST REPUBLIC OF SRI LANKA Humanitarian Operation—Factual Analysis TABLE OF CONTENTS Page I. EXECUTIVE SUMMARY 1 A. Overview of this Report 1 B. Overview of the Humanitarian Operation 1 PART ONE II. BACKGROUND 4 A. Overview of the LTTE 4 B. LTTE Atrocities against Civilians 6 C. Use of Child Soldiers by the LTTE 10 D. Ethnic Cleansing Carried out by the LTTE 10 E. Attacks on Democracy by the LTTE 11 F. The Global Threat posed by the LTTE 11 G. Proscription of the LTTE 12 III. SIZE AND SCOPE OF THE LTTE 13 A. Potency of the LTTE 13 B. Number of Cadres 14 C. Land Fighting Forces 14 D. The Sea Tiger Wing 17 E. The Air Tiger Wing 20 F. Black Tiger (Suicide) Wing 22 G. Intelligence Wing 22 H. Supply Network 23 I. International Support Mechanisms 25 J. International Criminal Network 27 – iii – Humanitarian Operation—Factual Analysis Page IV. GOVERNMENT EFFORTS FOR A NEGOTIATED SETTLEMENT 28 A. Overview 28 B. The Thimpu Talks – July to August 1985 29 C. The Indo-Lanka Accord – July 1987 30 D. Peace Talks – May 1989 to June 1990 32 E. Peace Talks – October 1994 to April 1995 33 F. Norwegian-Facilitated Peace Process – February 2002 to January 2008 35 G. LTTE Behaviour during 2002–2006 37 PART TWO V. RESUMPTION OF HOSTILITIES 43 VI. THE WANNI OPERATION 52 VII.