A Raman and Infrared Spectroscopic Analysis of the Phosphate Mineral
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Secondary Phosphate Minerals from Conselheiro Pena Pegmatite District (Minas Gerais, Brazil): Substitutions of Triphylite and Montebrasite Scholz, R.; Chaves, M
The secondary phosphate minerals from Conselheiro Pena Pegmatite District (Minas Gerais, Brazil): substitutions of triphylite and montebrasite Scholz, R.; Chaves, M. L. S. C.; Belotti, F. M.; Filho, M. Cândido; Filho, L. Autor(es): A. D. Menezes; Silveira, C. Publicado por: Imprensa da Universidade de Coimbra URL persistente: URI:http://hdl.handle.net/10316.2/31441 DOI: DOI:http://dx.doi.org/10.14195/978-989-26-0534-0_27 Accessed : 2-Oct-2021 20:21:49 A navegação consulta e descarregamento dos títulos inseridos nas Bibliotecas Digitais UC Digitalis, UC Pombalina e UC Impactum, pressupõem a aceitação plena e sem reservas dos Termos e Condições de Uso destas Bibliotecas Digitais, disponíveis em https://digitalis.uc.pt/pt-pt/termos. Conforme exposto nos referidos Termos e Condições de Uso, o descarregamento de títulos de acesso restrito requer uma licença válida de autorização devendo o utilizador aceder ao(s) documento(s) a partir de um endereço de IP da instituição detentora da supramencionada licença. Ao utilizador é apenas permitido o descarregamento para uso pessoal, pelo que o emprego do(s) título(s) descarregado(s) para outro fim, designadamente comercial, carece de autorização do respetivo autor ou editor da obra. Na medida em que todas as obras da UC Digitalis se encontram protegidas pelo Código do Direito de Autor e Direitos Conexos e demais legislação aplicável, toda a cópia, parcial ou total, deste documento, nos casos em que é legalmente admitida, deverá conter ou fazer-se acompanhar por este aviso. pombalina.uc.pt digitalis.uc.pt 9 789892 605111 Série Documentos A presente obra reúne um conjunto de contribuições apresentadas no I Congresso Imprensa da Universidade de Coimbra Internacional de Geociências na CPLP, que decorreu de 14 a 16 de maio de 2012 no Coimbra University Press Auditório da Reitoria da Universidade de Coimbra. -

New Mineral Names*
American Mineralogist, Volume 75, pages 240-246, 1990 NEW MINERAL NAMES* JOHN L. JAMBOR CANMET, 555 Booth Street, Ottawa, Ontario KIA OGI, Canada EDWARD S. GREW Department of Geological Sciences, University of Maine, Orono, Maine 04469, U.S.A. Baiyuneboite-(Ce) the formula for cordylite-(Ce) requires revision such that Pigqiu Fu, Xlanze Su (1987) Baiyuneboite-A new min- cordylite-(Ce) and baiyuneboite-(Ce) may be identical. The eral. Acta Mineralogica Sinica, 7, 289-297 (in Chinese, Chairman therefore withdrew the approval and asked that English abstract). the authors withhold publication of their description of Pingqiu Fu, Youhua Kong, Guohong Gong, Meicheng baiyuneboite-(Ce) until the matter could be resolved. The Shao, Jinzi Qian (1987) The crystal structure of bai- request, unfortunately, was ignored. J.L.J. yuneboite-(Ce). Acta Mineralogica Sinica, 7, 298-304 (in Chinese, English abstract). Diaoyudaoite* Electron-microprobe analyses of ten grains, whose va- lidity was checked by single-crystal X-ray methods prior Shunxi Shen, Lirong Chen, Anchun Li, Tailu Dong, Qiu- to analysis, gave an average ofNa20 4.73, CaO 1.04, BaO huo Huang, Wenqiang Xu (1986) Diaoyudaoite-A new 20.38, Ce20, 24.21, La20, 10.92, Pr20, 0.62, Nd20, 10.04, mineral. Acta Mineralogica Sinica, 6, 224-227 (in Gd20, 0.13, F 2.50, C02 (by gas chromatography) 24.64, Chinese, English abstract). o == F 1.05, sum 98.16 wt%, corresponding to Nal.OS- The average of 13 electron-microprobe analyses gave (BaO.94CaO.I,)n07( Ce I.OSLao.4sN do.42Pr 0.0'Gdo.OI)"1.99F 0.9'C,.97- Na20 4.54, AI20, 93.00, Cr20, 1.95, MgO 0.10, CaO 0.10, 012.07'ideally NaBaCe2F(CO')4' The mineral occurs as yel- SiOz 0.23, K20 0.12, sum 100.04 wt%, corresponding to low, irregular grains 0.3 to 3 mm in size, frequently as (N ao.87Ko.ozMgo.02CaO.01)ro.92(AllO.84CrO.lsSio.02)"'1.01 0,7, ide- thin hexagonal tablets. -

A Vibrational Spectroscopic Study of the Phosphate Mineral Vantasselite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 147 (2015) 185–192 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A vibrational spectroscopic study of the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O ⇑ Ray L. Frost a, , Ricardo Scholz b, Fernanda Maria Belotti c, Andrés López a, Frederick L. Theiss a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35,400-00, Brazil c Federal University of Itajubá, Campus Itabira, Itabira, MG 35,903-087, Brazil highlights graphical abstract We have studied the phosphate mineral vantasselite Al2(PO4)(OH)3Á3H2O. Using a combination of SEM with EDX and Raman and infrared spectroscopy. Chemical analysis shows a mineral containing Al, Fe and P. A comparison is made with other aluminum containing phosphate minerals. Vibrational spectroscopy offers a mechanism for the study of the molecular structure of vantasselite. article info abstract Article history: We have studied the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O using a combination of SEM Received 28 October 2014 with EDX and Raman and infrared spectroscopy. Qualitative chemical analysis shows Al, Fe and P. Received in revised form 15 January 2015 À1 3À Raman bands at 1013 and 1027 cm are assigned to the PO4 m1 symmetric stretching mode. The obser- Accepted 23 March 2015 vation of two bands suggests the non-equivalence of the phosphate units in the vantasselite structure. -

Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, the Fluorine Analog of Wardite from the Silver Coin Mine, Valmy, Nevada
American Mineralogist, Volume 99, pages 804–810, 2014 Fluorowardite, NaAl3(PO4)2(OH)2F22 Anthony R. KAmpf1,*, pAul m. AdAms2, RobeRt m. housley3 And GeoRGe R. RossmAn3 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A. 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, U.S.A. 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. AbstRAct Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analog of wardite, is a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, U.S.A., where it oc- curs as a low-temperature secondary mineral in complex phosphate assemblages rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal-pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated density is 2.760 g/cm3 analyses (average of 8) provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O52O 13.35 (structure), total 94.74 wt%. The presence of H2O and OH and the absence of CO3 3+ anions) is: (Na0.87Ca0.13Mg0.04)(Al2.96Fe0.04)(P1.96As0.03)O8.12(OH)2.35F1.53·2H2O. Fluorowardite 3 is tetragonal, P41212, acV , and Z lines in the X-ray powder diffraction pattern are [dobsI)(hkl)]: 4.766(100)(004,103); 3.099(75) (211,203); 3.008(62)(115,212); 2.834(28)(204,213); 2.597(56)(205); 1.7628(32)(400,401); 1.6592(29) R1FoF) 62O) octahedra, PO4 tetrahedra, and NaO6(H2O)26 octahedra link by corner-sharing to form a square array. -

Burangaite, a New Phosphate Mineral from Rwanda
BURANGAITE, A NEW PHOSPHATE MINERAL FROM RWANDA O. von KNORRING, MARTTI LEHTINEN and TH. G. SAHAMA KNORRING, O. von, LEHTINEN, MARTTI and SAHAMA, Th. G. 1977: Burangaite, a new phosphate mineral from Rwanda. BulL GeoL Soc. Finland 49: 33-36. This paper describes a new phosphate, burangaite, from the Buranga pegmatite in Rwanda. Burangaite is monoclinic with the idealized formula (Na,Ca)2 (Fe2+,Mg)2Aho(OH,Oh2(P04)S·4H20, Z = 2. The crystals exhibit narrow, bladed prisms, elongated parallel to the b-axis. Perfect cleavage parallel to 100. Mohs' hardness 5. Streak slightly bluish. 0 Unit-cell data: ao 25.09 A, bo 5.048 A, Co 13.45 A, fJ 110.91 , space group C2Ic. These parameters and the indexed X-ray powder pattern (Table 1) indicate a marked relationship with dufrenite. 0 The mineral is blue in color with y 11 band c /\a = 11 0, 2Va = 58 , strong pleochroism, refractive indices a 1.611, fJ 1.635, Y 1.643. Com mon hourglass structure with a blue core and a colorless margin. O. von Knorring, Department of Earth Sciences, Leeds University, Leeds LS2 9JT, England. Martti Lehtinen and Th. G. Sahama, Department of Geology and Mineralogy, University of Helsinki, P.O. Box 115, SF-00170 Hel sinki 17, Finland. Introduction commonly associated with bjarebyite (von Knorring and Fransolet 1975), wardite and The occurrence of a long-prismatic bluish other phosphates under study. Wardite occurs phosphate mineral from the Buranga pegma as white crystals of pyramidal habit, up to tite, Rwanda, was noted and provisionally 2 mm in size, with dominating {012} and described by one of us (von Knorring 1973). -

Roscherite-Group Minerals from Brazil
■ ■ Roscherite-Group Minerals yÜÉÅ UÜté|Ä Daniel Atencio* and José M.V. Coutinho Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. *e-mail: [email protected] Luiz A.D. Menezes Filho Rua Esmeralda, 534 – Prado, 30410-080 - Belo Horizonte, MG, Brazil. INTRODUCTION The three currently recognized members of the roscherite group are roscherite (Mn2+ analog), zanazziite (Mg analog), and greifensteinite (Fe2+ analog). These three species are monoclinic but triclinic variations have also been described (Fanfani et al. 1977, Leavens et al. 1990). Previously reported Brazilian occurrences of roscherite-group minerals include the Sapucaia mine, Lavra do Ênio, Alto Serra Branca, the Córrego Frio pegmatite, the Lavra da Ilha pegmatite, and the Pirineus mine. We report here the following three additional occurrences: the Pomarolli farm, Lavra do Telírio, and São Geraldo do Baixio. We also note the existence of a fourth member of the group, an as-yet undescribed monoclinic Fe3+-dominant species with higher refractive indices. The formulas are as follows, including a possible formula for the new species: Roscherite Ca2Mn5Be4(PO4)6(OH)4 • 6H2O Zanazziite Ca2Mg5Be4(PO4)6(OH)4 • 6H2O 2+ Greifensteinite Ca2Fe 5Be4(PO4)6(OH)4 • 6H2O 3+ 3+ Fe -dominant Ca2Fe 3.33Be4(PO4)6(OH)4 • 6H2O ■ 1 ■ Axis, Volume 1, Number 6 (2005) www.MineralogicalRecord.com ■ ■ THE OCCURRENCES Alto Serra Branca, Pedra Lavrada, Paraíba Unanalyzed “roscherite” was reported by Farias and Silva (1986) from the Alto Serra Branca granite pegmatite, 11 km southwest of Pedra Lavrada, Paraíba state, associated with several other phosphates including triphylite, lithiophilite, amblygonite, tavorite, zwieselite, rockbridgeite, huréaulite, phosphosiderite, variscite, cyrilovite and mitridatite. -
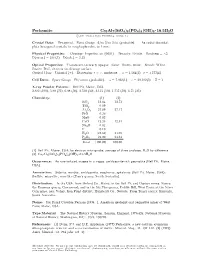
Perhamite Ca3al7(Sio4)3(PO4)4(OH)3 ² 16:5H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Hexagonal
Perhamite Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 6=m 2=m 2=m (probable). As radial discoidal, platy hexagonal crystals, in rough spherules, to 1 mm. Physical Properties: Cleavage: Imperfect on 0001 . Tenacity: Brittle. Hardness = 5 f g » D(meas.) = 2.64(1) D(calc.) = 2.53 Optical Properties: Translucent to nearly opaque. Color: Brown, white. Streak: White. Luster: Dull, vitreous on cleavage surface. Optical Class: Uniaxial (+). Dispersion: r > v; moderate. ! = 1.564(2) ² = 1.577(4) Cell Data: Space Group: P 6=mmm (probable). a = 7.022(1) c = 20.182(5) Z = 1 X-ray Powder Pattern: Bell Pit, Maine, USA. 2.882 (100), 5.80 (71), 6.08 (50), 3.510 (50), 3.115 (50), 1.757 (50), 6.71 (35) Chemistry: (1) (2) SiO2 13.64 13.72 TiO2 0.09 Al2O3 27.09 27.17 FeO 0.26 MgO 0.02 CaO 12.26 12.81 Na2O 0.02 F 0.10 H2O [24.62] 24.69 P2O5 21.90 21.61 Total [100.00] 100.00 (1) Bell Pit, Maine, USA; by electron microprobe, average of three analyses, H2O by di®erence. (2) Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O Occurrence: As rare isolated masses in a vuggy, amblygonite-rich pegmatite (Bell Pit, Maine, USA). Association: Siderite, wardite, amblygonite, eosphorite, sphalerite (Bell Pit, Maine, USA); °uellite, minyulite, wavellite (Tom's quarry, South Australia). Distribution: In the USA, from Oxford Co., Maine, in the Bell Pit and Dunton mines, Newry; the Emmons quarry, Greenwood; and in the Ski Pike quarry, Cobble Hill, West Paris; at the Silver Coin mine, near Valmy, Iron Point district, Humboldt Co., Nevada. -

Fluorowardite Naal3(PO4)2(OH)2F2·2H2O
Fluorowardite NaAl3(PO4)2(OH)2F2·2H2O Crystal Data: Tetragonal. Point Group: 422. As tetragonal bipyramidal crystals, to 0.1 mm, displaying {001} (prominent and lustrous), {011} and/or {012} (prominent, irregular, and striated parallel to [100]), and {100} (common, irregular, and striated parallel to [100]). Physical Properties: Cleavage: Perfect on {001}. Fracture: Irregular. Tenacity: Brittle. Hardness = 5 D(meas.) = n.d. D(calc.) = 2.706 Optical Properties: Transparent to translucent. Color: Colorless to white or cream-colored. Streak: White. Luster: Vitreous to pearly. Optical Class: Uniaxial (+). ω = 1.576(2) ε = 1.584(2) Cell Data: Space Group: P41212. a = 7.077(2) c = 19.227(3) Z = 4 X-ray Powder Pattern: Silver Coin mine, Valmy, Humboldt County, Nevada, USA. 4.766 (100), 3.099 (75), 3.008 (62), 2.597 (56), 1.5228 (49), 1.7628 (32), 1.6592 (29) Chemistry: (1) (2) Na2O 6.27 7.71 CaO 1.74 MgO 0.42 Al2O3 35.21 38.05 Fe2O3 0.72 P2O5 32.49 35.32 As2O5 0.64 F 6.76 9.45 -O = F2 2.85 3.98 H2O [13.35] 13.45 total 94.74 100.00 (1) Silver Coin mine, Valmy, Humboldt County, Nevada, USA; average of 8 electron microprobe analyses supplemented by FTIR and Raman spectroscopy, H2O calculated from the structure; 3+ corresponds to (Na0.87Ca0.13Mg0.04)Σ=1.04(Al2.96Fe 0.04)Σ=3.00(P1.96As0.03)Σ=1.99O8.12(OH)2.35F1.53·2H2O. (2) NaAl3(PO4)2(OH)2F2·2H2O. Occurrence: A secondary phase, in a F-rich secondary phosphate assemblage. -

A Specific Gravity Index for Minerats
A SPECIFICGRAVITY INDEX FOR MINERATS c. A. MURSKyI ern R. M. THOMPSON, Un'fuersityof Bri.ti,sh Col,umb,in,Voncouver, Canad,a This work was undertaken in order to provide a practical, and as far as possible,a complete list of specific gravities of minerals. An accurate speciflc cravity determination can usually be made quickly and this information when combined with other physical properties commonly leads to rapid mineral identification. Early complete but now outdated specific gravity lists are those of Miers given in his mineralogy textbook (1902),and Spencer(M,i,n. Mag.,2!, pp. 382-865,I}ZZ). A more recent list by Hurlbut (Dana's Manuatr of M,i,neral,ogy,LgE2) is incomplete and others are limited to rock forming minerals,Trdger (Tabel,l,enntr-optischen Best'i,mmungd,er geste,i,nsb.ildend,en M,ineral,e, 1952) and Morey (Encycto- ped,iaof Cherni,cal,Technol,ogy, Vol. 12, 19b4). In his mineral identification tables, smith (rd,entifi,cati,onand. qual,itatioe cherai,cal,anal,ys'i,s of mineral,s,second edition, New york, 19bB) groups minerals on the basis of specificgravity but in each of the twelve groups the minerals are listed in order of decreasinghardness. The present work should not be regarded as an index of all known minerals as the specificgravities of many minerals are unknown or known only approximately and are omitted from the current list. The list, in order of increasing specific gravity, includes all minerals without regard to other physical properties or to chemical composition. The designation I or II after the name indicates that the mineral falls in the classesof minerals describedin Dana Systemof M'ineralogyEdition 7, volume I (Native elements, sulphides, oxides, etc.) or II (Halides, carbonates, etc.) (L944 and 1951). -

A Study of the Phosphate Mineral Kapundaite Naca(Fe3+)4(PO4)4
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 122 (2014) 400–404 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa 3+ A study of the phosphate mineral kapundaite NaCa(Fe )4(PO4)4 (OH)3Á5(H2O) using SEM/EDX and vibrational spectroscopic methods ⇑ Ray L. Frost a, , Andrés López a, Yunfei Xi a, Ricardo Scholz b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35 400-00, Brazil highlights graphical abstract The kapundaite sample originated from the Sapucaia mine a lithium- bearing pegmatite. Kapundaite is a hydrated hydroxyl sodium calcium ferric phosphate 3+ NaCa(Fe )4(PO4)4(OH)3Á5(H2O). The molecular structure of kapundaite was assessed by vibrational spectroscopy. Multiple phosphate bands are observed. article info abstract Article history: Vibrational spectroscopy enables subtle details of the molecular structure of kapundaite to be deter- Received 17 September 2013 mined. Single crystals of a pure phase from a Brazilian pegmatite were used. Kapundaite is the Fe3+ mem- Received in revised form 5 November 2013 ber of the wardite group. The infrared and Raman spectroscopy were applied to compare the structure of Accepted 5 November 2013 kapundaite with wardite. The Raman spectrum of kapundaite in the 800–1400 cmÀ1 spectral range shows Available online 21 November 2013 À1 3À two intense bands at 1089 and 1114 cm assigned to the m1 PO4 symmetric stretching vibrations. -

Revision 1 1 2 Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, The
1 Revision 1 2 3 Fluorowardite, NaAl3(PO4)2(OH)2F2·2H2O, the fluorine analogue of wardite from the Silver Coin 4 mine, Valmy, Nevada. 5 1 2 3 3 6 ANTHONY R. KAMPF *, PAUL M. ADAMS , ROBERT M. HOUSLEY , AND GEORGE R. ROSSMAN 7 8 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition 9 Boulevard, Los Angeles, CA 90007, USA 10 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, USA 11 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, 12 CA 91125, USA 13 *Email: [email protected] 14 15 Abstract 16 Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analogue of wardite, is 17 a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, 18 USA, where it occurs as a low-temperature secondary mineral in complex phosphate assemblages 19 rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal- 20 pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to 21 translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, 22 the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated 23 density is 2.760 g/cm3. Optically, fluorowardite is uniaxial positive, with ω = 1.576(2) and ε = 24 1.584(2) (white light) and is non-pleochroic. Electron microprobe analyses (average of 8) 25 provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O5 0.64, F 26 6.76, O=F -2.85, H2O 13.35 (structure), total 94.74 wt%. -

The Crystal Structure of Warditei
MINERALOGICAL MAGAZINE, MARCH 1970, VOL. 37, NO. 289 The crystal structure of warditeI L. FANFANI, A. NUNZI, AND P. P. ZANAZZI Istituto di Mineralogia, Universita di Perugia, Italy SUMMARY. Wardite, NaAIa(OH).(POJ2. 2HzO, has a = 7.03 A, c = 19"04 A; space group P41212 or P4a212. Its crystal structure was solved by a three-dimensional Patterson function computed using intensity data photographically collected by the Weissenberg method, and refined by successive Fourier maps and least-squares cycles to a R index 0.062 for 3I 6 independent observed reflections. The wardite structure is formed by layers of AI and Na coordination polyhedra sharing vertices and edges. These sheets, parallel to the a axes, are connected to each other in the c direction by PO, tetrahedra and H-bonds. This structural feature accounts for the perfect {OOI} cleavage of wardite and explains the change that occurs in lattice parameters when Al is substituted by Fe in avelinoite. The relationships with the minerals of the trigonal families of crandaIlite, woodhouseite, and jarosite are also discussed. W ARDITE, first found by Davison (1896) in variscite nodules at Fairfield (Utah), occurs associated with millisite and crandallite, and was defined by Larsen and Shannon (1930) as a basic hydrated aluminum, sodium, and calcium phosphate. Pough (1937) described the morphology of the crystals and assigned them to the tetragonal system. Wardite was then discovered in pegmatitic rocks at Beryl Mountain, New Hampshire (Hurlbut, 1952), and assigned the formula NaAI3(OH)4(P04)2.2H20. It is likely that calcium replaces sodium to some extent and this could account for the earlier analyses of the mineral.