Technological Challenges of Phosphorus Removal in High-Phosphorus Ores: Sustainability Implications and Possibilities for Greener Ore Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Precipitation of Phosphate Minerals from Effluent of Anaerobically Digested Swine Manure Alex Y
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School January 2012 Precipitation of Phosphate Minerals from Effluent of Anaerobically Digested Swine Manure Alex Y. Lin University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the Chemical Engineering Commons, Environmental Engineering Commons, and the Environmental Sciences Commons Scholar Commons Citation Lin, Alex Y., "Precipitation of Phosphate Minerals from Effluent of Anaerobically Digested Swine Manure" (2012). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/4359 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Precipitation of Phosphate Minerals from Effluent of Anaerobically Digested Swine Manure by Alex Yuan-li Lin A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Civil and Environmental Engineering College of Engineering University of South Florida Co-Major Professor: Sarina Ergas, Ph.D. Co-Major Professor: Jeffrey Cunningham, Ph.D. Maya Trotz, Ph.D. Date of Approval: November 9, 2012 Keywords: struvite, wastewater, confined animal feeding operation (CAFO), fertilizer, synthetic Copyright © 2012, Alex Yuan-li Lin DEDICATION I dedicate this thesis to all those that have supported me along the way whether directly or indirectly. I would like to thank members of Intervarsity on the USF campus, members of Community Life Church, and my family for their support and encouragement in many ways. -

Nutrients: Nitrogen (N), Sulfur (S), Phosphorus (P), Potassium (K)
Nutrients: Nitrogen (N), Sulfur (S), Phosphorus (P), Potassium (K) Essential for all life, their availability (or lack thereof) controls the distribution of flora and fauna Look into their: Sources Pools (sinks) Fluxes root uptake + microbial uptake leaching solid solution Nutrients must be in a specific form – specie - for use by organisms Nutrient Cycling: N, S, P, K Soil Organic Matter (CHONPS) Minerals Primary Productivity (P, S, K) O Leaves & Roots A Decomposition Heterotrophic respiration B Gas loss SOM/Minerals Microbes leaching of nutrients plant nutrient uptake Nutrients: natural & anthropogenic sources (IN) and outputs (OUT) N2 fertilizers (chemical, manure, sludge) gases and particulates to atmosphere pesticides (fossil fuel combustion (coal/oil); cycles; trees) wet and dry deposition (acid rain, aerosols) harvesting plant tissue/residues root uptake IN OUT SOIL Pools (sinks) (SOM/clays/oxides) Fluxes (transformations) OUT IN ions and molecules in solution (leaching) roots (exudates, biomass) colloidal transport bedrock (1ry/2ry minerals in parent material) erosion runoff The Nitrogen Cycle + The Nitrogen Cycle Denitrification: - - NO3 is the most stable N2 (g) is prevalent in reduction of NO3 chemical form of N in the air of soil pores to reduced forms aerated soil solutions of N (N2) (energy- consuming) Atmospheric N deposition Nitrogen evolution (gases) Fertilizer additions 700 (+5) +5 Oxic - denitrification NO3 (+3) zone - NO2 (+2) (mV) NO (+1) h E N2O Suboxic (0) nitrification N state oxidation N2 236 zone immobilization -
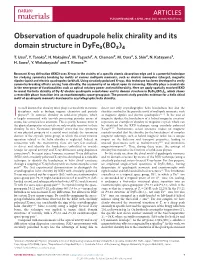
Observation of Quadrupole Helix Chirality and Its Domain Structure in Dyfe3(BO3)4
ARTICLES PUBLISHED ONLINE: 6 APRIL 2014 | DOI: 10.1038/NMAT3942 Observation of quadrupole helix chirality and its domain structure in DyFe3(BO3)4 T. Usui1, Y. Tanaka2, H. Nakajima1, M. Taguchi2, A. Chainani2, M. Oura2, S. Shin2, N. Katayama3, H. Sawa3, Y. Wakabayashi1 and T. Kimura1* Resonant X-ray diraction (RXD) uses X-rays in the vicinity of a specific atomic absorption edge and is a powerful technique for studying symmetry breaking by motifs of various multipole moments, such as electric monopoles (charge), magnetic dipoles (spin) and electric quadrupoles (orbital). Using circularly polarized X-rays, this technique has been developed to verify symmetry breaking eects arising from chirality, the asymmetry of an object upon its mirroring. Chirality plays a crucial role in the emergence of functionalities such as optical rotatory power and multiferroicity. Here we apply spatially resolved RXD to reveal the helix chirality of Dy 4f electric quadrupole orientations and its domain structure in DyFe3(BO3)4, which shows a reversible phase transition into an enantiomorphic space-group pair. The present study provides evidence for a helix chiral motif of quadrupole moments developed in crystallographic helix chirality. t is well known that chirality often plays a critical role in various detect not only crystallographic helix handedness but also the disciplines, such as biology, organic chemistry and particle chirality ascribed to the periodic motif of multipole moments, such Iphysics1,2. In contrast, chirality in solid-state physics, which as magnetic dipoles and electric quadrupoles12–14. In the case of is largely concerned with crystals possessing periodic arrays of magnetic dipoles, the handedness of a `helical magnetic structure' atoms, has attracted less attention. -

Biogeochemistry of Mediterranean Wetlands: a Review About the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions
water Review Biogeochemistry of Mediterranean Wetlands: A Review about the Effects of Water-Level Fluctuations on Phosphorus Cycling and Greenhouse Gas Emissions Inmaculada de Vicente 1,2 1 Departamento de Ecología, Universidad de Granada, 18071 Granada, Spain; [email protected]; Tel.: +34-95-824-9768 2 Instituto del Agua, Universidad de Granada, 18071 Granada, Spain Abstract: Although Mediterranean wetlands are characterized by extreme natural water level fluctu- ations in response to irregular precipitation patterns, global climate change is expected to amplify this pattern by shortening precipitation seasons and increasing the incidence of summer droughts in this area. As a consequence, a part of the lake sediment will be exposed to air-drying in dry years when the water table becomes low. This periodic sediment exposure to dry/wet cycles will likely affect biogeochemical processes. Unexpectedly, to date, few studies are focused on assessing the effects of water level fluctuations on the biogeochemistry of these ecosystems. In this review, we investigate the potential impacts of water level fluctuations on phosphorus dynamics and on greenhouse gases emissions in Mediterranean wetlands. Major drivers of global change, and specially water level fluctuations, will lead to the degradation of water quality in Mediterranean wetlands by increasing the availability of phosphorus concentration in the water column upon rewetting of dry sediment. CO2 fluxes are likely to be enhanced during desiccation, while inundation is likely to decrease cumulative CO emissions, as well as N O emissions, although increasing CH emissions. Citation: de Vicente, I. 2 2 4 Biogeochemistry of Mediterranean However, there exists a complete gap of knowledge about the net effect of water level fluctuations Wetlands: A Review about the Effects induced by global change on greenhouse gases emission. -

Vibrational Spectroscopic Characterization of the Phosphate Mineral Ludlamite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 103 (2013) 143–150 Contents lists available at SciVerse ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa Vibrational spectroscopic characterization of the phosphate mineral ludlamite (Fe,Mn,Mg)3(PO4)2Á4H2O – A mineral found in lithium bearing pegmatites ⇑ Ray L. Frost a, , Yunfei Xi a, Ricardo Scholz b, Fernanda M. Belotti c a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil c Federal University of Itajubá, Campus Itabira, Itabira, MG 35903-087, Brazil highlights graphical abstract " We have analyzed the phosphate Raman spectrum of ludlamite in the phosphate stretching region. mineral ludlamite by EMP-WDS. " The mineral is a ferrous phosphate with some minor substitution of Mg and Mn. " Spectroscopic analysis shows the mineral is predominantly a phosphate with some minor hydrogen phosphate units. " The position of the OH bands shows that water is very strongly hydrogen bonded in the ludlamite structure. article info abstract Article history: The objective of this work is to analyze ludlamite (Fe,Mn,Mg)3(PO4)2Á4H2O from Boa Vista mine, Galiléia, Available online 16 November 2012 Brazil and to assess the molecular structure of the mineral. The phosphate mineral ludlamite has been characterized by EMP-WDS, Raman and infrared spectroscopic measurements. The mineral is shown to Keywords: be a ferrous phosphate with some minor substitution of Mg and Mn. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

DESCRIPTIVE HUMAN PATHOLOGICAL MINERALOGY 1179 but Still Occursregularly
Amerkan Mincraloght, Volume 59, pages I177-1182, 1974 DescriptiveHuman Pathological Mineralogy Rrcneno I. Gmsox P.O. Box I O79, Dauis,C alilornia 95 6 I 6 Absfract Crystallographic, petrographic, and X-ray powder difiraction analysis of approximately 15,000 samples showed that the most common mineral constituents of human pathological concretions are calcium oxalates (whewellite and weddellite), calcium phosphates (apatite, brushite, and whitlockite), and magnesium phosphates (struvite and newberyite). Less are monetite, hannayite, calcite, aragonite, vaterite, halite, gypsum, and hexahydrite."o-rnon of the variables determining which minerals precipitate, the effects of different pH values on deposi- tional conditions are most apparent, and are shown by occurrences and relationships among many of the minerals studied. A pH-sensitive series has been identified among magnesium phosphatesin concretions. Introduction The study was carried out over a period of three The importanceof mineralogyin the field of medi- years.Composition was confirmedby X-ray powder cine lies in the applicationof mineralogicalmethods diffraction and polarizing microscopy;sequence was to study pathologicalmineral depositsin the human arrived at from considerationsof microscopic tex- body. Urology benefitsgreatly becauseconcretions tural and crystallographicrelationships. More than of mineral matter (calculi) are common in the 14,500samples were derivedfrom the urinary sys- urinary system.The value of mineralogicalanalysis tem of kidneys,ureters, bladder, and urethra; the of urinary material was first describedby prien and remaining samples are not statistically significant Frondel (1947). Mineralogistsmay be unawareof and arediscussed only briefly. the variability and nature of such compounds be- Calcium cause reports are usually published in medical Oxalates journals. This investigationreports the mineralogy Whewellite, CaCzOE.H2O,and weddellite, CaCz- and possiblepathological significanceof these min- O4'2H2O,are very uncommonin the mineralworld. -

SYMMETRY of PHOSPHOSIDERITE Duncelc Mcconnbr.L, The
SYMMETRY OF PHOSPHOSIDERITE DuNcelc McCoNNBr.l, The Uniaersity of Teras, Austin, Teras INrnooucuoN Prior to the description of crystals lrom Sardinia by M. de Angelis (l) in 1926, phosphosiderite was supposed to exhibit the external sym- metry of a substance belonging to the orthorhombic holohedral class. This mineral had been investigated by H. Laubmann and H. Steinmetz (2) as well as the authors who originally described the mineral, W. Bruhns and K. Busz (3). More recently phosphosideritehas been de- scribedfrom Kirunavara, Sweden,by R. Koechlin (4). De Angelis demonstrated that the symmetry is monoclinic on the basis of three difierent types of evidence: (1) The etch figures on the basal pinakoid do not exhibit planar symmetry with respect to a plane normal to the o axis, nor axial sym- metry with respect to the c axis (Fig. 4). (2) The goniometric measurementscannot be reconciled with ortho- rhombic axes of reference,but are quite amenable to monoclinic axes wittr B:89"24t. Certain prominent faces are not repeated as required by orthorhombic symmetry. (3) The optical data are not consistent with orthorhombic symmetry, becauseX Ac> J-3". Before the appearanceof the work by De Angelis, W. T. Schaller (5) had pointed out the marked similarity between the compositions,crystal habits and axial ratios of phosphosiderite and metavariscite [formerly called variscite (6)] and he had suggestedthe possibility of an isodimor- phous series: Octahedral Habit Tabular Habit Scorodite Strengite Phosphosiderite Variscite Metavariscite P. Kokkoros (7) has recently shown that scorodite and strengite are isostructural and the writer has found that variscite produces a powder diffraction pattern sufficiently similar to indicate that it also belongs to this group. -

The Global Marine Phosphorus Cycle: Sensitivity to Oceanic Circulation
Biogeosciences, 4, 155–171, 2007 www.biogeosciences.net/4/155/2007/ Biogeosciences © Author(s) 2007. This work is licensed under a Creative Commons License. The global marine phosphorus cycle: sensitivity to oceanic circulation C. P. Slomp and P. Van Cappellen Department of Earth Sciences – Geochemistry, Faculty of Geosciences, Utrecht University, P.O. Box 80021, 3508 TA Utrecht, The Netherlands Received: 4 September 2006 – Published in Biogeosciences Discuss.: 5 October 2006 Revised: 8 January 2007 – Accepted: 20 February 2007 – Published: 22 February 2007 Abstract. A new mass balance model for the coupled ma- stand long-term variations in marine biological activity, at- rine cycles of phosphorus (P) and carbon (C) is used to ex- mospheric composition and climate (Holland, 1984; Van amine the relationships between oceanic circulation, primary Cappellen and Ingall, 1996; Petsch and Berner, 1998; Bjer- productivity, and sedimentary burial of reactive P and partic- rum and Canfield, 2002). Important forcings include the sup- ulate organic C (POC), on geological time scales. The model ply of reactive P from the continents, oceanic circulation and explicitly represents the exchanges of water and particulate sea level fluctuations (Follmi,¨ 1996; Compton et al., 2000; matter between the continental shelves and the open ocean, Handoh and Lenton, 2003; Wallmann, 2003; Bjerrum et al., and it accounts for the redox-dependent burial of POC and 2006). the various forms of reactive P (iron(III)-bound P, particu- Upward transport of nutrient-rich water sustains biologi- late organic P (POP), authigenic calcium phosphate, and fish cal activity in marine surface waters. Vertical mixing, how- debris). Steady state and transient simulations indicate that ever, also controls the ventilation of the deeper ocean waters, a slowing down of global ocean circulation decreases pri- which in turn has a major effect on the sedimentary burial mary production in the open ocean, but increases that in the of phosphorus. -
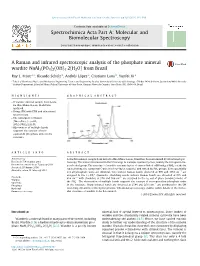
A Raman and Infrared Spectroscopic Analysis of the Phosphate Mineral
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 126 (2014) 164–169 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A Raman and infrared spectroscopic analysis of the phosphate mineral wardite NaAl3(PO4)2(OH)4Á2(H2O) from Brazil ⇑ Ray L. Frost a, , Ricardo Scholz b, Andrés López a, Cristiano Lana b, Yunfei Xi a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil was analysed. Using SEM with EDX and vibrational spectroscopy. The calculated formula is (Na0.97Ca0.03)R1.00Al3 (PO4)2(OH)4Á2(H2O). Observation of multiple bands supports the concept of non- equivalent phosphate units in the structure. article info abstract Article history: A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil has been examined by vibrational spec- Received 17 November 2013 troscopy. The mineral is unusual in that it belongs to a unique symmetry class, namely the tetragonal-tra- Received in revised form 7 January 2014 pezohedral group. The structure of wardite contains layers of corner-linked –OH bridged MO6 octahedra Accepted 2 February 2014 stacked along the tetragonal C-axis in a four-layer sequence and linked by PO groups. Consequentially Available online 15 February 2014 4 not all phosphate units are identical. -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

2015 AFMS Endowment Fund Drawing
2015 AFMS Endowment Fund Drawing The 2014 Endowment Fund Drawing took place in Tulsa, Oklahoma. We had some very nice donations for prizes, and a lot of people stepped up and bought tickets to support the AFMS Endowment Fund. Thank you for your support. And thank you to those who picked up prizes to deliver at the banquet in Tulsa. That helped save the cost of mailing the items. We have received the following donations for the 2015 Endowment Fund Drawing, which will be held on October 24, 2015, at the awards banquet in Austin, Texas. 1.Copper piece and stock certificate donated by Pam Hecht. Estimated value is $65. MWF 2. Howelite and onyx agate necklace and earrings. Donated by Sharon Rogow, crafted by Betsy Oberheim. Estimated value is $75. CFMS 2015 AFMS Endowment Fund Drawing Page 1 of 11 10-11-2015 3. A large agatized coral head donated by the Suncoast Gem and Mineral Society. It was from an old collection of Withlacoochee River coral donated by a member who passed some years ago. The value is estimated to be at least $100. SFMS (This one is larger than the one given last year.) 4. Phareodus encaustus (fossil fish) from the Eocene Period, Green River formation, Kemmerer, Wyoming. This was donated by J.C. and Donna Moore with an approximate value of $65. MWF #5 - is a Tampa Bay Coral pair donated by Barbara Sky, MWF Uniform Rules Chairman. The pair is about six inches long and two and a half inches wide. The estimated value is $50.