A Vibrational Spectroscopic Study of the Phosphate Mineral Whiteite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A Raman and Infrared Spectroscopic Analysis of the Phosphate Mineral
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 126 (2014) 164–169 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A Raman and infrared spectroscopic analysis of the phosphate mineral wardite NaAl3(PO4)2(OH)4Á2(H2O) from Brazil ⇑ Ray L. Frost a, , Ricardo Scholz b, Andrés López a, Cristiano Lana b, Yunfei Xi a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil was analysed. Using SEM with EDX and vibrational spectroscopy. The calculated formula is (Na0.97Ca0.03)R1.00Al3 (PO4)2(OH)4Á2(H2O). Observation of multiple bands supports the concept of non- equivalent phosphate units in the structure. article info abstract Article history: A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil has been examined by vibrational spec- Received 17 November 2013 troscopy. The mineral is unusual in that it belongs to a unique symmetry class, namely the tetragonal-tra- Received in revised form 7 January 2014 pezohedral group. The structure of wardite contains layers of corner-linked –OH bridged MO6 octahedra Accepted 2 February 2014 stacked along the tetragonal C-axis in a four-layer sequence and linked by PO groups. Consequentially Available online 15 February 2014 4 not all phosphate units are identical. -

A Vibrational Spectroscopic Study of the Phosphate Mineral Vantasselite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 147 (2015) 185–192 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A vibrational spectroscopic study of the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O ⇑ Ray L. Frost a, , Ricardo Scholz b, Fernanda Maria Belotti c, Andrés López a, Frederick L. Theiss a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35,400-00, Brazil c Federal University of Itajubá, Campus Itabira, Itabira, MG 35,903-087, Brazil highlights graphical abstract We have studied the phosphate mineral vantasselite Al2(PO4)(OH)3Á3H2O. Using a combination of SEM with EDX and Raman and infrared spectroscopy. Chemical analysis shows a mineral containing Al, Fe and P. A comparison is made with other aluminum containing phosphate minerals. Vibrational spectroscopy offers a mechanism for the study of the molecular structure of vantasselite. article info abstract Article history: We have studied the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O using a combination of SEM Received 28 October 2014 with EDX and Raman and infrared spectroscopy. Qualitative chemical analysis shows Al, Fe and P. Received in revised form 15 January 2015 À1 3À Raman bands at 1013 and 1027 cm are assigned to the PO4 m1 symmetric stretching mode. The obser- Accepted 23 March 2015 vation of two bands suggests the non-equivalence of the phosphate units in the vantasselite structure. -

New Mineral Names*,†
American Mineralogist, Volume 106, pages 1360–1364, 2021 New Mineral Names*,† Dmitriy I. Belakovskiy1, and Yulia Uvarova2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia In this issue This New Mineral Names has entries for 11 new species, including 7 minerals of jahnsite group: jahnsite- (NaMnMg), jahnsite-(NaMnMn), jahnsite-(CaMnZn), jahnsite-(MnMnFe), jahnsite-(MnMnMg), jahnsite- (MnMnZn), and whiteite-(MnMnMg); lasnierite, manganflurlite (with a new data for flurlite), tewite, and wumuite. Lasnierite* the LA-ICP-MS analysis, but their concentrations were below detec- B. Rondeau, B. Devouard, D. Jacob, P. Roussel, N. Stephant, C. Boulet, tion limits. The empirical formula is (Ca0.59Sr0.37)Ʃ0.96(Mg1.42Fe0.54)Ʃ1.96 V. Mollé, M. Corre, E. Fritsch, C. Ferraris, and G.C. Parodi (2019) Al0.87(P2.99Si0.01)Ʃ3.00(O11.41F0.59)Ʃ12 based on 12 (O+F) pfu. The strongest lines of the calculated powder X-ray diffraction pattern are [dcalc Å (I%calc; Lasnierite, (Ca,Sr)(Mg,Fe)2Al(PO4)3, a new phosphate accompany- ing lazulite from Mt. Ibity, Madagascar: an example of structural hkl)]: 4.421 (83; 040), 3.802 (63, 131), 3.706 (100; 022), 3.305 (99; 141), characterization from dynamic refinement of precession electron 2.890 (90; 211), 2.781 (69; 221), 2.772 (67; 061), 2.601 (97; 023). It diffraction data on submicrometer sample. European Journal of was not possible to perform powder nor single-crystal X-ray diffraction Mineralogy, 31(2), 379–388. -

Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, the Fluorine Analog of Wardite from the Silver Coin Mine, Valmy, Nevada
American Mineralogist, Volume 99, pages 804–810, 2014 Fluorowardite, NaAl3(PO4)2(OH)2F22 Anthony R. KAmpf1,*, pAul m. AdAms2, RobeRt m. housley3 And GeoRGe R. RossmAn3 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A. 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, U.S.A. 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. AbstRAct Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analog of wardite, is a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, U.S.A., where it oc- curs as a low-temperature secondary mineral in complex phosphate assemblages rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal-pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated density is 2.760 g/cm3 analyses (average of 8) provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O52O 13.35 (structure), total 94.74 wt%. The presence of H2O and OH and the absence of CO3 3+ anions) is: (Na0.87Ca0.13Mg0.04)(Al2.96Fe0.04)(P1.96As0.03)O8.12(OH)2.35F1.53·2H2O. Fluorowardite 3 is tetragonal, P41212, acV , and Z lines in the X-ray powder diffraction pattern are [dobsI)(hkl)]: 4.766(100)(004,103); 3.099(75) (211,203); 3.008(62)(115,212); 2.834(28)(204,213); 2.597(56)(205); 1.7628(32)(400,401); 1.6592(29) R1FoF) 62O) octahedra, PO4 tetrahedra, and NaO6(H2O)26 octahedra link by corner-sharing to form a square array. -

Roscherite-Group Minerals from Brazil
■ ■ Roscherite-Group Minerals yÜÉÅ UÜté|Ä Daniel Atencio* and José M.V. Coutinho Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. *e-mail: [email protected] Luiz A.D. Menezes Filho Rua Esmeralda, 534 – Prado, 30410-080 - Belo Horizonte, MG, Brazil. INTRODUCTION The three currently recognized members of the roscherite group are roscherite (Mn2+ analog), zanazziite (Mg analog), and greifensteinite (Fe2+ analog). These three species are monoclinic but triclinic variations have also been described (Fanfani et al. 1977, Leavens et al. 1990). Previously reported Brazilian occurrences of roscherite-group minerals include the Sapucaia mine, Lavra do Ênio, Alto Serra Branca, the Córrego Frio pegmatite, the Lavra da Ilha pegmatite, and the Pirineus mine. We report here the following three additional occurrences: the Pomarolli farm, Lavra do Telírio, and São Geraldo do Baixio. We also note the existence of a fourth member of the group, an as-yet undescribed monoclinic Fe3+-dominant species with higher refractive indices. The formulas are as follows, including a possible formula for the new species: Roscherite Ca2Mn5Be4(PO4)6(OH)4 • 6H2O Zanazziite Ca2Mg5Be4(PO4)6(OH)4 • 6H2O 2+ Greifensteinite Ca2Fe 5Be4(PO4)6(OH)4 • 6H2O 3+ 3+ Fe -dominant Ca2Fe 3.33Be4(PO4)6(OH)4 • 6H2O ■ 1 ■ Axis, Volume 1, Number 6 (2005) www.MineralogicalRecord.com ■ ■ THE OCCURRENCES Alto Serra Branca, Pedra Lavrada, Paraíba Unanalyzed “roscherite” was reported by Farias and Silva (1986) from the Alto Serra Branca granite pegmatite, 11 km southwest of Pedra Lavrada, Paraíba state, associated with several other phosphates including triphylite, lithiophilite, amblygonite, tavorite, zwieselite, rockbridgeite, huréaulite, phosphosiderite, variscite, cyrilovite and mitridatite. -
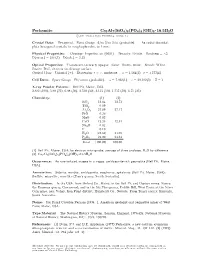
Perhamite Ca3al7(Sio4)3(PO4)4(OH)3 ² 16:5H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Hexagonal
Perhamite Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 6=m 2=m 2=m (probable). As radial discoidal, platy hexagonal crystals, in rough spherules, to 1 mm. Physical Properties: Cleavage: Imperfect on 0001 . Tenacity: Brittle. Hardness = 5 f g » D(meas.) = 2.64(1) D(calc.) = 2.53 Optical Properties: Translucent to nearly opaque. Color: Brown, white. Streak: White. Luster: Dull, vitreous on cleavage surface. Optical Class: Uniaxial (+). Dispersion: r > v; moderate. ! = 1.564(2) ² = 1.577(4) Cell Data: Space Group: P 6=mmm (probable). a = 7.022(1) c = 20.182(5) Z = 1 X-ray Powder Pattern: Bell Pit, Maine, USA. 2.882 (100), 5.80 (71), 6.08 (50), 3.510 (50), 3.115 (50), 1.757 (50), 6.71 (35) Chemistry: (1) (2) SiO2 13.64 13.72 TiO2 0.09 Al2O3 27.09 27.17 FeO 0.26 MgO 0.02 CaO 12.26 12.81 Na2O 0.02 F 0.10 H2O [24.62] 24.69 P2O5 21.90 21.61 Total [100.00] 100.00 (1) Bell Pit, Maine, USA; by electron microprobe, average of three analyses, H2O by di®erence. (2) Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O Occurrence: As rare isolated masses in a vuggy, amblygonite-rich pegmatite (Bell Pit, Maine, USA). Association: Siderite, wardite, amblygonite, eosphorite, sphalerite (Bell Pit, Maine, USA); °uellite, minyulite, wavellite (Tom's quarry, South Australia). Distribution: In the USA, from Oxford Co., Maine, in the Bell Pit and Dunton mines, Newry; the Emmons quarry, Greenwood; and in the Ski Pike quarry, Cobble Hill, West Paris; at the Silver Coin mine, near Valmy, Iron Point district, Humboldt Co., Nevada. -

Fluorowardite Naal3(PO4)2(OH)2F2·2H2O
Fluorowardite NaAl3(PO4)2(OH)2F2·2H2O Crystal Data: Tetragonal. Point Group: 422. As tetragonal bipyramidal crystals, to 0.1 mm, displaying {001} (prominent and lustrous), {011} and/or {012} (prominent, irregular, and striated parallel to [100]), and {100} (common, irregular, and striated parallel to [100]). Physical Properties: Cleavage: Perfect on {001}. Fracture: Irregular. Tenacity: Brittle. Hardness = 5 D(meas.) = n.d. D(calc.) = 2.706 Optical Properties: Transparent to translucent. Color: Colorless to white or cream-colored. Streak: White. Luster: Vitreous to pearly. Optical Class: Uniaxial (+). ω = 1.576(2) ε = 1.584(2) Cell Data: Space Group: P41212. a = 7.077(2) c = 19.227(3) Z = 4 X-ray Powder Pattern: Silver Coin mine, Valmy, Humboldt County, Nevada, USA. 4.766 (100), 3.099 (75), 3.008 (62), 2.597 (56), 1.5228 (49), 1.7628 (32), 1.6592 (29) Chemistry: (1) (2) Na2O 6.27 7.71 CaO 1.74 MgO 0.42 Al2O3 35.21 38.05 Fe2O3 0.72 P2O5 32.49 35.32 As2O5 0.64 F 6.76 9.45 -O = F2 2.85 3.98 H2O [13.35] 13.45 total 94.74 100.00 (1) Silver Coin mine, Valmy, Humboldt County, Nevada, USA; average of 8 electron microprobe analyses supplemented by FTIR and Raman spectroscopy, H2O calculated from the structure; 3+ corresponds to (Na0.87Ca0.13Mg0.04)Σ=1.04(Al2.96Fe 0.04)Σ=3.00(P1.96As0.03)Σ=1.99O8.12(OH)2.35F1.53·2H2O. (2) NaAl3(PO4)2(OH)2F2·2H2O. Occurrence: A secondary phase, in a F-rich secondary phosphate assemblage. -

2014-10-28 John Betts Fine Minerals
David Barthelmy From: John Betts <[email protected]> Sent: Tuesday, October 28, 2014 10:31 AM To: [email protected] Subject: New minerals are now online I returned from the Munich mineral show and new minerals were added to my web site. The minerals are now available for viewing at these 2 pages: New Listings Page 1a New Listings Page 1b or http://www.johnbetts-fineminerals.com/jhbnyc/newlist.htm http://www.johnbetts-fineminerals.com/jhbnyc/newlist1.htm Highlights of this week's minerals: - Copper (crystallized) from Emke Mine, Onganja, Namibia - Cinnabar, Chalcopyrite, Dolomite from Gortdrum Mine, Ireland - Pyromorphite from Bunker Hill Mine, 14th Level, Brown Vein, Idaho - Cuprite with Chrysocolla from Mupine Mine, Democratic Republic of the Congo - Cornetite with Malachite from Kalabi Mine, Democratic Republic of the Congo - Malachite from Lubumbashi, Democratic Republic of the Congo - Prehnite pseudomorphs after Laumontite with Apophyllite from Mumbai District, India - Narsarsukite from Poudrette Quarry, Mont Saint-Hilaire, Canada - Whiteite-(CaFeMg) with Quartz from Rapid Creek, Canada - Pyromorphite from Bad Ems District, Germany - Cassiterite with Quartz from Tenkergin Mine, Russia - Proustite from Schacht 207, Niederschlema, Germany - Calcite on Fluorite from Wuzhou, China - Beryl var. Aquamarine with Muscovite from Shigar Valley, Skardu District, Pakistan - Beryl var. Aquamarine from Mimoso do Sul, Brazil - Chrysocolla on Calcite with Chalcocite from Chimney Rock Quarry, New Jersey - Calcite (twinned crystals) -

A Study of the Phosphate Mineral Kapundaite Naca(Fe3+)4(PO4)4
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 122 (2014) 400–404 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa 3+ A study of the phosphate mineral kapundaite NaCa(Fe )4(PO4)4 (OH)3Á5(H2O) using SEM/EDX and vibrational spectroscopic methods ⇑ Ray L. Frost a, , Andrés López a, Yunfei Xi a, Ricardo Scholz b a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35 400-00, Brazil highlights graphical abstract The kapundaite sample originated from the Sapucaia mine a lithium- bearing pegmatite. Kapundaite is a hydrated hydroxyl sodium calcium ferric phosphate 3+ NaCa(Fe )4(PO4)4(OH)3Á5(H2O). The molecular structure of kapundaite was assessed by vibrational spectroscopy. Multiple phosphate bands are observed. article info abstract Article history: Vibrational spectroscopy enables subtle details of the molecular structure of kapundaite to be deter- Received 17 September 2013 mined. Single crystals of a pure phase from a Brazilian pegmatite were used. Kapundaite is the Fe3+ mem- Received in revised form 5 November 2013 ber of the wardite group. The infrared and Raman spectroscopy were applied to compare the structure of Accepted 5 November 2013 kapundaite with wardite. The Raman spectrum of kapundaite in the 800–1400 cmÀ1 spectral range shows Available online 21 November 2013 À1 3À two intense bands at 1089 and 1114 cm assigned to the m1 PO4 symmetric stretching vibrations. -

Revision 1 1 2 Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, The
1 Revision 1 2 3 Fluorowardite, NaAl3(PO4)2(OH)2F2·2H2O, the fluorine analogue of wardite from the Silver Coin 4 mine, Valmy, Nevada. 5 1 2 3 3 6 ANTHONY R. KAMPF *, PAUL M. ADAMS , ROBERT M. HOUSLEY , AND GEORGE R. ROSSMAN 7 8 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition 9 Boulevard, Los Angeles, CA 90007, USA 10 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, USA 11 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, 12 CA 91125, USA 13 *Email: [email protected] 14 15 Abstract 16 Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analogue of wardite, is 17 a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, 18 USA, where it occurs as a low-temperature secondary mineral in complex phosphate assemblages 19 rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal- 20 pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to 21 translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, 22 the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated 23 density is 2.760 g/cm3. Optically, fluorowardite is uniaxial positive, with ω = 1.576(2) and ε = 24 1.584(2) (white light) and is non-pleochroic. Electron microprobe analyses (average of 8) 25 provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O5 0.64, F 26 6.76, O=F -2.85, H2O 13.35 (structure), total 94.74 wt%. -

The Crystal Structure of Warditei
MINERALOGICAL MAGAZINE, MARCH 1970, VOL. 37, NO. 289 The crystal structure of warditeI L. FANFANI, A. NUNZI, AND P. P. ZANAZZI Istituto di Mineralogia, Universita di Perugia, Italy SUMMARY. Wardite, NaAIa(OH).(POJ2. 2HzO, has a = 7.03 A, c = 19"04 A; space group P41212 or P4a212. Its crystal structure was solved by a three-dimensional Patterson function computed using intensity data photographically collected by the Weissenberg method, and refined by successive Fourier maps and least-squares cycles to a R index 0.062 for 3I 6 independent observed reflections. The wardite structure is formed by layers of AI and Na coordination polyhedra sharing vertices and edges. These sheets, parallel to the a axes, are connected to each other in the c direction by PO, tetrahedra and H-bonds. This structural feature accounts for the perfect {OOI} cleavage of wardite and explains the change that occurs in lattice parameters when Al is substituted by Fe in avelinoite. The relationships with the minerals of the trigonal families of crandaIlite, woodhouseite, and jarosite are also discussed. W ARDITE, first found by Davison (1896) in variscite nodules at Fairfield (Utah), occurs associated with millisite and crandallite, and was defined by Larsen and Shannon (1930) as a basic hydrated aluminum, sodium, and calcium phosphate. Pough (1937) described the morphology of the crystals and assigned them to the tetragonal system. Wardite was then discovered in pegmatitic rocks at Beryl Mountain, New Hampshire (Hurlbut, 1952), and assigned the formula NaAI3(OH)4(P04)2.2H20. It is likely that calcium replaces sodium to some extent and this could account for the earlier analyses of the mineral. -

Whiteite-(Camgmg) Camg3al2(PO4)4(OH)2·8H2O
Whiteite-(CaMgMg) CaMg3Al2(PO4)4(OH)2·8H2O - - Crystal Data: Monoclinic. Point Group: 2/m. Crystals display {100}, {010}, {131 }, {111 }, and {001} as tapered blades, elongated along [100], flattened on {001}in radial fans. Twinning: By reflection on {001} common. Physical Properties: Cleavage: Perfect on {001}. Tenacity: Brittle. Fracture: Stepped irregular. Hardness = ~ 4 D(meas.) = 2.48(1) D(calc.) = 2.477 Optical Properties: Transparent. Color: Colorless. Streak: White. Luster: Vitreous. Optical Class: Biaxial (+). α = 1.564 β = 1.565 γ = 1.575 2V(meas.) = 24.1° 2V(calc.) = 35.3° Orientation: X = b; Z ^ a = 41° in obtuse β. No dispersion or pleochroism were observed. Cell Data: Space Group: P21/a. a = 14.8237(19) b = 7.0302(3) с = 9.946(3) β = 110.115(12)° Z = 2 X-ray Powder Pattern: Northern Belle mine, Candelaria district, Mineral County, Nevada, USA. 2.805 (100), 9.20 (82), 4.88 (64), 2.849 (45), 2.936 (40), 3.510 (35), 1.9527 (35) Chemistry: (1) (2) CaO 8.18 7.74 MgO 16.47 16.68 FeO 0.13 Al2O3 13.35 14.06 P2O5 38.84 39.16 H2O [22.32] 22.36 Total 99.29 100.00 (1) Northern Belle mine, Candelaria district, Mineral County, Nevada, USA; average of 7 electron microprobe analyses supplemented by Raman and FTIR spectroscopy, H2O calculated from 2+ structure; corresponds to Ca1.07Mg2.99Fe 0.01Al1.91P4O26H18.11. (2) CaMg3Al2(PO4)4(OH)2·8H2O. Mineral Group: Jahnsite group, whiteite subgroup. Occurrence: A low-temperature secondary mineral presumed to have formed as a result of hydrothermal alteration of phosphate nodules derived from the sediments.