Whiteite-(Camnmg) Camn Mg2al2(PO4)4(OH)2 • 8H2O C 2001-2005 Mineral Data Publishing, Version 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Mineral Names*,†
American Mineralogist, Volume 106, pages 1360–1364, 2021 New Mineral Names*,† Dmitriy I. Belakovskiy1, and Yulia Uvarova2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia In this issue This New Mineral Names has entries for 11 new species, including 7 minerals of jahnsite group: jahnsite- (NaMnMg), jahnsite-(NaMnMn), jahnsite-(CaMnZn), jahnsite-(MnMnFe), jahnsite-(MnMnMg), jahnsite- (MnMnZn), and whiteite-(MnMnMg); lasnierite, manganflurlite (with a new data for flurlite), tewite, and wumuite. Lasnierite* the LA-ICP-MS analysis, but their concentrations were below detec- B. Rondeau, B. Devouard, D. Jacob, P. Roussel, N. Stephant, C. Boulet, tion limits. The empirical formula is (Ca0.59Sr0.37)Ʃ0.96(Mg1.42Fe0.54)Ʃ1.96 V. Mollé, M. Corre, E. Fritsch, C. Ferraris, and G.C. Parodi (2019) Al0.87(P2.99Si0.01)Ʃ3.00(O11.41F0.59)Ʃ12 based on 12 (O+F) pfu. The strongest lines of the calculated powder X-ray diffraction pattern are [dcalc Å (I%calc; Lasnierite, (Ca,Sr)(Mg,Fe)2Al(PO4)3, a new phosphate accompany- ing lazulite from Mt. Ibity, Madagascar: an example of structural hkl)]: 4.421 (83; 040), 3.802 (63, 131), 3.706 (100; 022), 3.305 (99; 141), characterization from dynamic refinement of precession electron 2.890 (90; 211), 2.781 (69; 221), 2.772 (67; 061), 2.601 (97; 023). It diffraction data on submicrometer sample. European Journal of was not possible to perform powder nor single-crystal X-ray diffraction Mineralogy, 31(2), 379–388. -

Roscherite-Group Minerals from Brazil
■ ■ Roscherite-Group Minerals yÜÉÅ UÜté|Ä Daniel Atencio* and José M.V. Coutinho Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. *e-mail: [email protected] Luiz A.D. Menezes Filho Rua Esmeralda, 534 – Prado, 30410-080 - Belo Horizonte, MG, Brazil. INTRODUCTION The three currently recognized members of the roscherite group are roscherite (Mn2+ analog), zanazziite (Mg analog), and greifensteinite (Fe2+ analog). These three species are monoclinic but triclinic variations have also been described (Fanfani et al. 1977, Leavens et al. 1990). Previously reported Brazilian occurrences of roscherite-group minerals include the Sapucaia mine, Lavra do Ênio, Alto Serra Branca, the Córrego Frio pegmatite, the Lavra da Ilha pegmatite, and the Pirineus mine. We report here the following three additional occurrences: the Pomarolli farm, Lavra do Telírio, and São Geraldo do Baixio. We also note the existence of a fourth member of the group, an as-yet undescribed monoclinic Fe3+-dominant species with higher refractive indices. The formulas are as follows, including a possible formula for the new species: Roscherite Ca2Mn5Be4(PO4)6(OH)4 • 6H2O Zanazziite Ca2Mg5Be4(PO4)6(OH)4 • 6H2O 2+ Greifensteinite Ca2Fe 5Be4(PO4)6(OH)4 • 6H2O 3+ 3+ Fe -dominant Ca2Fe 3.33Be4(PO4)6(OH)4 • 6H2O ■ 1 ■ Axis, Volume 1, Number 6 (2005) www.MineralogicalRecord.com ■ ■ THE OCCURRENCES Alto Serra Branca, Pedra Lavrada, Paraíba Unanalyzed “roscherite” was reported by Farias and Silva (1986) from the Alto Serra Branca granite pegmatite, 11 km southwest of Pedra Lavrada, Paraíba state, associated with several other phosphates including triphylite, lithiophilite, amblygonite, tavorite, zwieselite, rockbridgeite, huréaulite, phosphosiderite, variscite, cyrilovite and mitridatite. -

2014-10-28 John Betts Fine Minerals
David Barthelmy From: John Betts <[email protected]> Sent: Tuesday, October 28, 2014 10:31 AM To: [email protected] Subject: New minerals are now online I returned from the Munich mineral show and new minerals were added to my web site. The minerals are now available for viewing at these 2 pages: New Listings Page 1a New Listings Page 1b or http://www.johnbetts-fineminerals.com/jhbnyc/newlist.htm http://www.johnbetts-fineminerals.com/jhbnyc/newlist1.htm Highlights of this week's minerals: - Copper (crystallized) from Emke Mine, Onganja, Namibia - Cinnabar, Chalcopyrite, Dolomite from Gortdrum Mine, Ireland - Pyromorphite from Bunker Hill Mine, 14th Level, Brown Vein, Idaho - Cuprite with Chrysocolla from Mupine Mine, Democratic Republic of the Congo - Cornetite with Malachite from Kalabi Mine, Democratic Republic of the Congo - Malachite from Lubumbashi, Democratic Republic of the Congo - Prehnite pseudomorphs after Laumontite with Apophyllite from Mumbai District, India - Narsarsukite from Poudrette Quarry, Mont Saint-Hilaire, Canada - Whiteite-(CaFeMg) with Quartz from Rapid Creek, Canada - Pyromorphite from Bad Ems District, Germany - Cassiterite with Quartz from Tenkergin Mine, Russia - Proustite from Schacht 207, Niederschlema, Germany - Calcite on Fluorite from Wuzhou, China - Beryl var. Aquamarine with Muscovite from Shigar Valley, Skardu District, Pakistan - Beryl var. Aquamarine from Mimoso do Sul, Brazil - Chrysocolla on Calcite with Chalcocite from Chimney Rock Quarry, New Jersey - Calcite (twinned crystals) -

Whiteite-(Camgmg) Camg3al2(PO4)4(OH)2·8H2O
Whiteite-(CaMgMg) CaMg3Al2(PO4)4(OH)2·8H2O - - Crystal Data: Monoclinic. Point Group: 2/m. Crystals display {100}, {010}, {131 }, {111 }, and {001} as tapered blades, elongated along [100], flattened on {001}in radial fans. Twinning: By reflection on {001} common. Physical Properties: Cleavage: Perfect on {001}. Tenacity: Brittle. Fracture: Stepped irregular. Hardness = ~ 4 D(meas.) = 2.48(1) D(calc.) = 2.477 Optical Properties: Transparent. Color: Colorless. Streak: White. Luster: Vitreous. Optical Class: Biaxial (+). α = 1.564 β = 1.565 γ = 1.575 2V(meas.) = 24.1° 2V(calc.) = 35.3° Orientation: X = b; Z ^ a = 41° in obtuse β. No dispersion or pleochroism were observed. Cell Data: Space Group: P21/a. a = 14.8237(19) b = 7.0302(3) с = 9.946(3) β = 110.115(12)° Z = 2 X-ray Powder Pattern: Northern Belle mine, Candelaria district, Mineral County, Nevada, USA. 2.805 (100), 9.20 (82), 4.88 (64), 2.849 (45), 2.936 (40), 3.510 (35), 1.9527 (35) Chemistry: (1) (2) CaO 8.18 7.74 MgO 16.47 16.68 FeO 0.13 Al2O3 13.35 14.06 P2O5 38.84 39.16 H2O [22.32] 22.36 Total 99.29 100.00 (1) Northern Belle mine, Candelaria district, Mineral County, Nevada, USA; average of 7 electron microprobe analyses supplemented by Raman and FTIR spectroscopy, H2O calculated from 2+ structure; corresponds to Ca1.07Mg2.99Fe 0.01Al1.91P4O26H18.11. (2) CaMg3Al2(PO4)4(OH)2·8H2O. Mineral Group: Jahnsite group, whiteite subgroup. Occurrence: A low-temperature secondary mineral presumed to have formed as a result of hydrothermal alteration of phosphate nodules derived from the sediments. -

A Vibrational Spectroscopic Study of the Phosphate Mineral Whiteite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 124 (2014) 243–248 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A vibrational spectroscopic study of the phosphate mineral ++ whiteite CaMn Mg2Al2(PO4)4(OH)2Á8(H2O) ⇑ Ray L. Frost a, , Ricardo Scholz b, Andrés López a, Yunfei Xi a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG, 35,400-00, Brazil highlights graphical abstract Vibrational spectroscopy enables subtle details of the molecular structure of whiteite. Crystals of a pure phase from a Brazilian pegmatite were used. Raman and infrared bands are assigned to the vibrations of 3À PO4 . A comparison is made with the vibrational spectra of wardite. article info abstract Article history: Vibrational spectroscopy enables subtle details of the molecular structure of whiteite to be determined. Received 8 October 2013 Single crystals of a pure phase from a Brazilian pegmatite were used. The infrared and Raman spectros- Received in revised form 17 December 2013 copy were applied to compare the molecular structure of whiteite with that of other phosphate minerals. Accepted 10 January 2014 À1 3À The Raman spectrum of whiteite shows an intense band at 972 cm assigned to the m1 PO4 symmetric Available online 21 January 2014 À1 3À stretching vibrations. The low intensity Raman bands at 1076 and 1173 cm are assigned to the m3 PO4 antisymmetric stretching modes. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

New Mineral Names*
American Mineralogist, Volume 75, pages 931-937, 1990 NEW MINERAL NAMES* JOHN L. JAMBOR CANMET, 555 Booth Street, Ottawa, Ontario KIA OGI, Canada EDWARD S. GREW Department of Geological Sciences, University of Maine, Orono, Maine 04469, U.S.A. Akhtenskite* Electron-microprobe analyses (using a JXA-50A probe F.V. Chukhrov, A.I. Gorshkov, A.V. Sivtsov, V.V. Be~- for Au and Sb, and a JXA-5 probe for 0) of one aggregate ezovskaya, YU.P. Dikov, G.A. Dubinina, N.N. Van- gave Au 49.7, 50.1, 49.3, Sb 39.2, 39.1, 39.2, 0 10.7, nov (1989) Akhtenskite- The natural analog of t-Mn02. 11.2, 11.2, sum 99.6, 100.4, 99.7 wt%; a second aggregate Izvestiya Akad. Nauk SSSR, ser. geol., No.9, 75-80 gave Au 52.4, 52.6, Sb 36.7, 36.3, 0 11.6, 11.4, sum (in Russian, English translation in Internat. Geol. Rev., 100.7, 100.3 wt%; the averages correspond to Au- 31, 1068-1072, 1989). F.V. Chukhrov, A.I. Gorshkov, V.S. Drits (1987) Ad- Sb1.2602.76and AU1.02Sblls02.83, respectively, close to a vances in the crystal chemistry of manganese oxides. theoretical composition AuSb03. H = 223.8 and 186.8 Zapiski Vses. Mineralog. Obshch. 16, 210-221 (in Rus- kg/mm2. No crystallographic parameters could be de- sian, English translation in Internat. Geol. Rev., 29, duced from the X-ray powder pattern, in which the fol- 434-444, 1987). lowing lines were present: 4.18( 100), 3.92(20), 3.72(30), 3.12(10), 2.08(10), 2.03(30), 1. -

Rittmannite, a New Mineral Species of the Whiteite Group from the Mangualde Granitic Pegmatite, Portugal
Canadian Mineralogist Vol. 27, pp. 447-449 (1989) RITTMANNITE, A NEW MINERAL SPECIES OF THE WHITEITE GROUP FROM THE MANGUALDE GRANITIC PEGMATITE, PORTUGAL YVONNE MARZONI FECIA DI CaSSATa AND PAOLO ORLANDI Dipartimento di Scienze delta Terra, Universita di Pisa, Via S. Maria 53, 56126 Pisa, Italy mov ANNA VEZZALINI Istituto di Mineralogia Petrografia, Universita di Modena, Via S. Eufemia 19, 41100 Modena, Italy ABSTRACT la formule (Mno.S4Cao.47b.OlMn1.oo(FeI.~sMno.S6Mgo.29) r;2.oo(Al1.7sFeo.2s)r;2.oo(OHh.02(P04)4.8H20. Densite mesu- Rittmannite, a new mineral species, is the MnMnFe ree 2.81(1), et calculee pour Z = 2, 2.83. La rittmannite analog of whiteite. It was found in altered phosphatic "nod- est biaxe positive. CXmes1.622, (3eale 1.626, 'Yeale 1.654, 2 V roes wes" from the Mangualde granitic pegmatite, Portugal, 43(2)°; I'orientation de I'indicatrice optique est: Xllb, Z:e as very small (0.3 x 0.3 x 0.04 mm), pale yellow, tabu- + 7°. Aucun pleochroYsme n'a ete decele. Le nom honore lar, pseudohexagonal crystals forming aggregates of sub- M. Ie professeur Alfred Rittman (1893-1980), volcanolo- parallel individual crystals. It occurs on kryzhanovskite, gue de renommee mondiale qui a contribue au developpe- frondeIite, hureaulite and adularia. Rittmannite is ment de la mineralogie, la petrographie et la microscopie monoclixyc, space group P2la, a !5.01(4), b 6.89(3), e optique. 10.16(3) A, (3 112.82(25)°, V 968(4) A3. The strongest lines (Traduit par la Redaction) in the X-ray powder-diffraction pattern [d in A(Iob,)(hkl)] are: 9.38(8)(001), 4.69(m8)(002), 2.802(8)(222). -
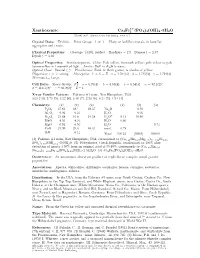
Xanthoxenite Ca4fe (PO4)
3+ • Xanthoxenite Ca4Fe2 (PO4)4(OH)2 3H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Triclinic. Point Group: 1or1. Platy or lathlike crystals, in lamellar aggregates and crusts. Physical Properties: Cleavage: {010}, perfect. Hardness = 2.5 D(meas.) = 2.97 D(calc.) = 3.38 Optical Properties: Semitransparent. Color: Pale yellow, brownish yellow; pale yellow to pale lemon-yellow in transmitted light. Luster: Dull to slightly waxy. Optical Class: Biaxial (–). Pleochroism: Faint in thick grains; in shades of yellow. Dispersion: r< v,strong. Absorption: Y > X = Z. α = 1.704(3) β = 1.715(3) γ = 1.724(3) 2V(meas.) = Large. Cell Data: Space Group: P 1. a = 6.70(4) b = 8.85(4) c = 6.54(3) α =92.1(2)◦ β = 110.1(2)◦ γ =93.2(2)◦ Z=1 X-ray Powder Pattern: Palermo #1 mine, New Hampshire, USA. 3.05 (10), 2.73 (9), 3.22 (8), 3.48 (7), 2.23 (6), 6.24 (5), 4.94 (4) Chemistry: (1) (2) (3) (1) (2) (3) P2O5 37.62 38.1 38.37 Na2O 0.10 Al2O3 0.01 0.23 K2O 0.05 + Fe2O3 21.68 16.6 21.58 H2O 9.13 10.90 − MnO 4.55 4.10 H2O 0.86 MgO 0.91 0.50 H2O 9.74 CaO 24.99 29.3 30.31 insol. 0.78 SrO 0.11 Total 100.53 [100.0] 100.00 (1) Palermo #1 mine, New Hampshire, USA; corresponds to (Ca3.28Mn0.47Mg0.17)Σ=3.92Fe2.00 • (PO4)3.90(OH)2.14 2.69H2O. -

Whiteite-(Mnfemg) Mg2al2(PO4)4(OH)2 • 8H2O C 2001-2005 Mineral Data Publishing, Version 1
(Mn2+, Ca)(Fe2+, Mn2+) Whiteite-(MnFeMg) Mg2Al2(PO4)4(OH)2 • 8H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals are warped, canoe-shaped, showing only {111}, {001}, to 1.5 cm. Twinning: By reflection on {001}, common, giving a pseudo-orthorhombic outline. Physical Properties: Cleavage: On {001}, good to perfect. Hardness = 3–4 D(meas.) = 2.67(2) D(calc.) = 2.62 Optical Properties: Semitransparent. Color: Chocolate-brown; colorless in thin fragments. Optical Class: Biaxial (+). Orientation: X ⊥{001}. α = 1.575(5) β = 1.585(5) γ = 1.595(5) 2V(meas.) = 80◦–90◦ ◦ 0 Cell Data: Space Group: P 21/a. a = 14.99(2) b = 6.96(1) c = 10.14(1) β = 113 19(6) Z=2 X-ray Powder Pattern: Lavra da Ilha pegmatite; close to whiteite-(CaFeMg). 9.318 (100), 2.776 (90), 4.824 (50), 2.948 (45), 4.644 (40), 3.518 (35), 3.245 (35) Chemistry: (1) P2O5 36.4 Al2O3 12.7 FeO 7.9 MnO 7.6 MgO 10.1 CaO 1.4 H2O n.d. Total (1) Lavra da Ilha pegmatite, Brazil; by electron probe, partial analysis, total Fe as FeO, total 2+ 2+ 2+ Mn as MnO; corresponding approximately to (Mn0.9Ca0.2)Σ=1.1(Fe0.9Mn0.1)Σ=1.0Mg2.0Al2.0 • (PO4)4(OH)2 8H2O. Mineral Group: Whiteite group; Al > Fe3+ in the M(3) structural site. Occurrence: In complex zoned granite pegmatites. Association: Eosphorite, zanazziite, wardite, albite, quartz (Lavra da Ilha pegmatite, Brazil). Distribution: From the Lavra da Ilha pegmatite, in the Jequitinhonha River, three km north of Taquaral, and at the Enio´ pegmatite mine, northeast of Galil´eia,Minas Gerais, Brazil. -

X-Ray Structure Refinement and Vibrational Spectroscopy Of
crystals Article X-ray Structure Refinement and Vibrational Spectroscopy of Metavauxite FeAl2(PO4)2(OH)2·8H2O Giancarlo Della Ventura 1,2 , Francesco Capitelli 3, Giancarlo Capitani 4, Gennaro Ventruti 5,* and Alessandro Monno 5 1 Dipartimento di Scienze, Università Roma Tre, Largo S. L. Murialdo 1, 00146 Rome, Italy; [email protected] 2 Istituto Nazionale di Fisica Nucleare, Via E. Fermi 40, I-00044 Rome, Italy 3 Institute of Crystallography-CNR, Via Salaria Km 29.300, 00016 Monterotondo (Roma), Italy; [email protected] 4 Dipartimento di Scienze dell’Ambiente e della Terra (DISAT), Piazza della Scienza 4, 20126 Milano, Italy; [email protected] 5 Dipartimento di Scienze della Terra e Geoambientali, Università di Bari, via E. Orabona 4, 70125 Bari, Italy; [email protected] * Correspondence: [email protected]; Tel.: +39-080-5442596 Received: 11 May 2019; Accepted: 3 June 2019; Published: 6 June 2019 Abstract: In this paper, we provide a crystal-chemical investigation of metavauxite, ideally FeAl (PO ) (OH) 8H O, from Llallagua (Bolivia) by using a multi-methodological approach based 2 4 2 2· 2 on EDS microchemical analysis, single crystal X-ray diffraction, and Raman and Fourier transform infrared (FTIR) spectroscopy. Our new diffraction results allowed us to locate all hydrogen atoms from the structure refinements in the monoclinic P21/c space group. Metavauxite structure displays 7 a complex framework consisting of a stacking of [Al(PO4)3(OH)(H2O)2] − layers linked to isolated 2+ [Fe(H2O)6] cationic octahedral complex solely by hydrogen bonding. The hydrogen-bonding scheme was inferred from bond-valence calculations and donor-acceptor distances. -

The Discovery of New Mineral Species and Type Minerals from Brazil a Descoberta De Novas Espécies Minerais E Minerais Tipo Do Brasil
DOI: 10.1590/23174889201500010011 INVITED REVIEW The discovery of new mineral species and type minerals from Brazil A descoberta de novas espécies minerais e minerais tipo do Brasil Daniel Atencio1* RESUMO: Os minerais foram vistos apenas como fontes de produtos ABSTRACT: Minerals were seen merely as sources of chemicals: químicos: minério de ferro, minério de cobre, etc. No entanto, não são iron ore, copper ore, etc. However, minerals are not just chemicals apenas associações de elementos químicos, uma vez que apresentam es- associations, since they display crystal structures. These two features truturas cristalinas. Essas duas características em conjunto proporcionam together provide properties that can be technologically useful. Even propriedades que podem ser tecnologicamente úteis. Mesmo que um though a mineral occurs in very small amount, which does not al- mineral ocorra em quantidade muito pequena, o que não permite a sua low its extraction, it can serve as a model for obtaining the synthetic extração, pode servir como um modelo para a obtenção do análogo sin- analogue on an industrial scale. It is necessary that a new-mineral tético em uma escala industrial. É necessário que uma proposta de novo proposal be submitted for approval by the Commission on New Min- mineral seja submetida à aprovação pela Comissão de Novos Minerais, erals, Nomenclature and Classification (CNMNC) of the Interna- Nomenclatura e Classificação (CNMNC) da Associação Mineralógica tional Mineralogical Association (IMA) before publication. Only 65 Internacional (IMA) antes da publicação. Somente 65 espécies minerais valid mineral species were first described from Brazil, that is, the type válidas foram descritas pela primeira vez no Brasil, isto é, minerais-tipo minerals from Brazil.