The Metric System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

RAD 520-3 the Physics of Medical Dosimetry
RAD 520 The Physics of Medical Dosimetry I Fall Semester Syllabus COURSE DEFINITION: RAD 520-3 The Physics of Medical Dosimetry I- This course covers the following topics: Radiologic Physics, production of x-rays, radiation treatment and simulation machines, interactions of ionizing radiation, radiation measurements, dose calculations, computerized treatment planning, dose calculation algorithms, electron beam characteristics, and brachytherapy physics and procedures. This course is twenty weeks in length. Prerequisite: Admission to the Medical Dosimetry Program. COURSE OBJECTIVES: 1. Demonstrate an understanding of radiation physics for photons and electrons. 2. Demonstrate an understanding of the different types of radiation production. 3. Demonstrate an understanding of radiation dose calculations and algorithms. 4. Understand brachytherapy procedures and calculate radiation attenuation and decay. 5. Demonstrate an understanding of the different types of radiation detectors. 6. Demonstrate an understanding of general treatment planning. COURSE OUTLINE: Topics 1. Radiation physics 2. Radiation generators 3. External beam calculations 4. Brachytherapy calculations 5. Treatment planning 6. Electron beam physics COURSE REQUIREMENTS: Purchase all texts, attend all lectures, and complete required examinations, quizzes, and homeworks. Purchase a T130XA scientific calculator. PREREQUISITES: Admittance to the Medical Dosimetry Program. TEXTBOOKS: Required: 1. Khan, F. M. (2014). The physics of radiation therapy (5th ed.). Philadelphia: Wolters Kluwer 2. Khan, F.M. (2016). Treatment planning in radiation oncology (4th ed.). Philadelphia: Wolters Kluwer 3. Washington, C. M., & Leaver, D. T. (2015). Principles and practices of radiation therapy (4th Ed). St. Louis: Mosby. Optional: (Students typically use clinical sites’ copy) 1. Bentel, G. C. (1992). Radiation therapy planning (2nd ed.). New York: McGraw-Hill. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united. -
Metric System.Pdf
METRIC SYSTEM THE METRIC SYSTEM The metric system is much easier. All metric units are related by factors of 10. Nearly the entire world (95%), except the United States, now uses the metric system. Metric is used exclusively in science. Because the metric system uses units related by factors of ten and the types of units (distance, area, volume, mass) are simply-related, performing calculations with the metric system is much easier. METRIC CHART Prefix Symbol Factor Number Factor Word Kilo K 1,000 Thousand Hecto H 100 Hundred Deca Dk 10 Ten Base Unit Meter, gram, liter 1 One Deci D 0.1 Tenth Centi C 0.01 Hundredth Milli M 0.001 Thousandth The metric system has three units or bases. Meter – the basic unit used to measure length Gram – the basic unit used to measure weight Liter – the basic unit used to measure liquid capacity (think 2 Liter cokes!) The United States, Liberia and Burma (countries in black) have stuck with using the Imperial System of measurement. You can think of “the metric system” as a nickname for the International System of Units, or SI. HOW TO REMEMBER THE PREFIXES Kids Kilo Have Hecto Dropped Deca Over base unit (gram, liter, meter) Dead Deci Converting Centi Metrics Milli Large Units – Kilo (1000), Hecto (100), Deca (10) Small Units – Deci (0.1), Centi (0.01), Milli (0.001) Because you are dealing with multiples of ten, you do not have to calculate anything. All you have to do is move the decimal point, but you need to understand what you are doing when you move the decimal point. -

Colorado Metric Conversion Manual
COLORADO OT . DEPARTMENT OF TRANSPORTATION METRIC CONVERSION MANUAL January 1994 This manual or any part thereof must not be reproduced in any form without the following disclaimer. The information presented in this publication haS been prepared in accordance with recognized engineering principles and is for general information only. While it is believed to be accurate, this information should not be used or relied upon for any specific application without competent professional examination and verification of its accuracy, suitability, and applicability by a competent licensed engineer or other licensed professional. Publication of the material contained herein is not intended as a representation or warranty on the part of the Colorado Department of Transportation (CDOT) , that this information is suitable for any general or particular use or of freedom from infringement of any patent or patents. Anyone making use of this information assumes all liability arising from such use. Caution must be exercised when relying upon the specifications and codes developed by other bodies and incorporated herein, since such material may be modified or amended from time to time subsequent to the printing of this edition. COOT bears no responsibility for such material other than to incorporate it at the time of the initial publication of this edition, subject to the general comments set forth in the preceding paragraph. Table of Contents Preface ................................................... v Introduction . vii Chapter 1: Metric Units, Terms, Symbols, and Conversion Factors . ... 1-1 Basic Metric . 1-1 Length, Area, Volume and Temperature . 1-7 Civil and Structural Engineering . 1-10 Metric Project Definition ....................... .. 1-12 Chapter 2: Right-Of-Way ................................... -
Conversion Factors A
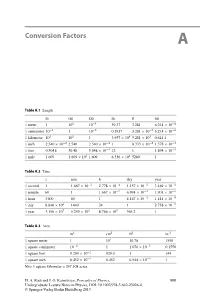
Conversion Factors A Table A.1 Length mcmkmin.ftmi 1 meter 1 102 10−3 39.37 3.281 6.214 × 10−4 1 centimeter 10−2 110−5 0.3937 3.281 × 10−2 6.214 × 10−6 1 kilometer 103 105 13.937 × 104 3.281 × 103 0.621 4 1inch 2.540 × 10−2 2.540 2.540 × 10−5 18.333 × 10−2 1.578 × 10−5 1 foot 0.3048 30.48 3.048 × 10−4 12 1 1.894 × 10−4 1 mile 1 609 1.609 × 105 1.609 6.336 × 104 5280 1 Table A.2 Time s min h day year 1 second 1 1.667 × 10−2 2.778 × 10−4 1.157 × 10−5 3.169 × 10−8 1 minute 60 1 1.667 × 10−2 6.994 × 10−4 1.901 × 10−6 1 hour 3600 60 1 4.167 × 10−2 1.141 × 10−4 1day 8.640 × 104 1440 24 1 2.738 × 10−5 1 year 3.156 × 107 5.259 × 105 8.766 × 103 365.2 1 Table A.3 Area m2 cm2 ft2 in.2 1 square meter 1 104 10.76 1550 1 square centimeter 10−4 11.076 × 10−3 0.1550 1 square foot 9.290 × 10−2 929.0 1 144 1 square inch 6.452 × 10−4 6.452 6.944 × 10−3 1 Note 1 square kilometer = 247.108 acres H. A. Radi and J. O. Rasmussen, Principles of Physics, 999 Undergraduate Lecture Notes in Physics, DOI: 10.1007/978-3-642-23026-4, © Springer-Verlag Berlin Heidelberg 2013 1000 A Conversion Factors Table A.4 Volume m3 cm3 Lft3 in.3 1 cubic meter 1 106 1000 35.51 6.102 × 104 1 cubic centimeter 10−6 11.000 × 10−3 3.531 × 10−5 6.102 × 10−2 1liter 1.000 × 10−3 1000 1 3.531 × 10−2 61.02 1 cubic foot 2.832 × 10−4 1 28.32 1 1728 1 cubic inch 1.639 × 10−4 16.39 1.639 × 10−2 5.787 × 10−4 1 Note 1 U.S. -

The Fantastic Family Becquerel the Radiant
art & radiation exhibition entitled “Did you say Radiation The Fantastic Family Protection? Stories of X-rays, radioactivity, the etc.” is an absorbing display of artistic Becquerel interpretations of the concepts of radiation and radiation This work pays tribute to a dynasty of great minds, the protection. Dealing with a complex subject, the artists Becquerels: Antoine Cesar, the grandfather; Edmond, the entice the audience to formulate new opinions about radiation protection through their works. father; Henri, the son; all renowned physicists. It is a special tribute to Henri Becquerel who, following in his father’s foot- The exhibits stimulate visitors to interact with these steps, became France’s expert on luminescence. scientific concepts at a sensorial level rather than rationally trying to explain them. As visitors are drawn through these displays, they are forced to confront these artworks Artist: Peter Keene engaging all their senses: touch, sight, hearing, smell and taste. The artists aim to educate the visitor about the key elements that surround the subject of radiation, such as the difference between X-rays and radioactivity, the context in which radiation was discovered, the hazard it poses and how to protect one self from it. This combination of science and art is a travelling exhibition co-produced by the Institute for Radiation Protection and Nuclear Safety (France), Montbéliard Science Pavilion (France) and the Pays de Montbéliard Metropolitan Region (France); The Curie Museum (Paris), the Röntgen Museum (Remscheid, Germany) and the Deutsches Museum- (Munich, Germany), also contributed to the exhibition. The exhibition travelled around France, then to Buenos Aires, Argentina. In the future it is schedule to go on display The Radiant One in Lusanne, Switzerland (late 2009-early 2010) and Helsiniki, A mysterious vessel in the shape of an atom-smasher Finland (2010), before returning to France. -

The International System of Units (SI) - Conversion Factors For
NIST Special Publication 1038 The International System of Units (SI) – Conversion Factors for General Use Kenneth Butcher Linda Crown Elizabeth J. Gentry Weights and Measures Division Technology Services NIST Special Publication 1038 The International System of Units (SI) - Conversion Factors for General Use Editors: Kenneth S. Butcher Linda D. Crown Elizabeth J. Gentry Weights and Measures Division Carol Hockert, Chief Weights and Measures Division Technology Services National Institute of Standards and Technology May 2006 U.S. Department of Commerce Carlo M. Gutierrez, Secretary Technology Administration Robert Cresanti, Under Secretary of Commerce for Technology National Institute of Standards and Technology William Jeffrey, Director Certain commercial entities, equipment, or materials may be identified in this document in order to describe an experimental procedure or concept adequately. Such identification is not intended to imply recommendation or endorsement by the National Institute of Standards and Technology, nor is it intended to imply that the entities, materials, or equipment are necessarily the best available for the purpose. National Institute of Standards and Technology Special Publications 1038 Natl. Inst. Stand. Technol. Spec. Pub. 1038, 24 pages (May 2006) Available through NIST Weights and Measures Division STOP 2600 Gaithersburg, MD 20899-2600 Phone: (301) 975-4004 — Fax: (301) 926-0647 Internet: www.nist.gov/owm or www.nist.gov/metric TABLE OF CONTENTS FOREWORD.................................................................................................................................................................v -

Radiation: Units of Measure and Health Effects Gerald Gels Health Physicist Veridian Corporation Units of Measure
Radiation: Units of Measure and Health Effects Gerald Gels Health Physicist Veridian Corporation Units of Measure • Traditional Unit 1 curie (Ci) = 3.7 x 1010 dps = 2.2 x 1012 dpm • Subunits 1 microcurie (µCi) = 1 x 10-6 Ci 1 picocurie (pCi) = 1 x 10-12 Ci • International System (SI) 1 becquerel (Bq) = 1 dps Ionization Density alpha m = 4; Z = +2 beta m = .0005; Z = -1 gamma m = 0; Z = 0 m = atomic mass units (amu) Z = electric charge units = ionizations roentgen (R): An amount of x- or gamma radiation that causes 1 esu (electrostatic unit) of charges due to ionization in 1 cc of air. Absorbed Dose The rad (r) [or Gray (Gy)] G. L. Gels The Roentgen (R) describes the radiation field (the agent); but, The rad (r) describes the effect in a medium. 1 rad = 100 ergs/gm 100 ergs of energy released per gram of medium a. applies to any medium (including air) b. applies to any type of radiation (not just photons) The Gray (Gy) = 100 rad (r) The rad is a medium-dependent quantity, and is very useful as an estimate of the effect of radiation in, say, tissue. However, it does not take into account the relative biological effects of different types of radiation. 1 rad = 0.87 R For air 1 rad = 0.98 R For soft tissue Dose Equivalent [rem or Seivert (Sv)] * Gamma rays have a much different biological effect than alpha particles * The Dose Equivalent modifies the absorbed dose (rad) by the relative biological effectiveness (or, Quality Factor, QF) of the radiation Radiation QF -rays X-rays } above .03 MeV 1 less than .03 MeV 1.7 Neutrons (thermal) 2 Neutrons (fast) Protons } 10 Alpha particles Heavy charged particles } 20 rem = rad x QF G. -

RAD H2O Manual, Much of Which Is Incorporated in This Version
RAD H2O Radon in Water Accessory for the RAD7 User Manual INTRODUCTION Te RAD H2O is an accessory to the RAD7 that enables you to measure radon in water over a concentration range of from less than 10 pCi/L to greater than 400,000 pCi/L. By diluting your sample, or by waiting for sample decay, you can extend the method's upper range to any concentration. Te equipment is portable and battery operated, and the measurement is fast. You can have an accurate reading of radon in water within an hour of taking the sample. Te RAD H2O gives results afer a 30 minutes analysis with a sensitivity that matches or exceeds that of liquid scintillation methods. Te method is simple and straightforward. Tere are no harmful chemicals to use. Once the procedure becomes familiar and well understood it will produce accurate results with minimal effort. It is assumed that the user has a good, working knowledge of the RAD7. If both the RAD7 and the RAD H2O are new to the user, then time should be spent learning how to make good measurements of radon in air with the RAD7 before embarking on radon in water measurements. Instructions for RAD7 operation with the RAD H2O are given in this manual but, for more detail about the instrument and its use, the reader is referred to the RAD7 manual. Grateful acknowledgment is made of the significant contribution to this manual by Stephen Shefsky, who wrote most of the original NITON RAD H2O manual, much of which is incorporated in this version. -

Recommended Practice for the Use of Metric (SI) Units in Building Design and Construction NATIONAL BUREAU of STANDARDS
<*** 0F ^ ££v "ri vt NBS TECHNICAL NOTE 938 / ^tTAU Of U.S. DEPARTMENT OF COMMERCE/ 1 National Bureau of Standards ^^MMHHMIB JJ Recommended Practice for the Use of Metric (SI) Units in Building Design and Construction NATIONAL BUREAU OF STANDARDS 1 The National Bureau of Standards was established by an act of Congress March 3, 1901. The Bureau's overall goal is to strengthen and advance the Nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research and provides: (1) a basis for the Nation's physical measurement system, (2) scientific and technological services for industry and government, (3) a technical basis for equity in trade, and (4) technical services to pro- mote public safety. The Bureau consists of the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, the Institute for Computer Sciences and Technology, the Office for Information Programs, and the Office of Experimental Technology Incentives Program. THE ENSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consist- ent system of physical measurement; coordinates that system with measurement systems of other nations; and furnishes essen- tial services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of the Office of Measurement Services, and the following center and divisions: Applied Mathematics — Electricity