Changing Metric Units
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metric System Units of Length
Math 0300 METRIC SYSTEM UNITS OF LENGTH Þ To convert units of length in the metric system of measurement The basic unit of length in the metric system is the meter. All units of length in the metric system are derived from the meter. The prefix “centi-“means one hundredth. 1 centimeter=1 one-hundredth of a meter kilo- = 1000 1 kilometer (km) = 1000 meters (m) hecto- = 100 1 hectometer (hm) = 100 m deca- = 10 1 decameter (dam) = 10 m 1 meter (m) = 1 m deci- = 0.1 1 decimeter (dm) = 0.1 m centi- = 0.01 1 centimeter (cm) = 0.01 m milli- = 0.001 1 millimeter (mm) = 0.001 m Conversion between units of length in the metric system involves moving the decimal point to the right or to the left. Listing the units in order from largest to smallest will indicate how many places to move the decimal point and in which direction. Example 1: To convert 4200 cm to meters, write the units in order from largest to smallest. km hm dam m dm cm mm Converting cm to m requires moving 4 2 . 0 0 2 positions to the left. Move the decimal point the same number of places and in the same direction (to the left). So 4200 cm = 42.00 m A metric measurement involving two units is customarily written in terms of one unit. Convert the smaller unit to the larger unit and then add. Example 2: To convert 8 km 32 m to kilometers First convert 32 m to kilometers. km hm dam m dm cm mm Converting m to km requires moving 0 . -

Lesson 1: Length English Vs
Lesson 1: Length English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer B. 1 yard or 1 meter C. 1 inch or 1 centimeter English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 yard = 0.9444 meters English vs. Metric Units Which is longer? A. 1 mile or 1 kilometer 1 mile B. 1 yard or 1 meter C. 1 inch or 1 centimeter 1.6 kilometers 1 inch = 2.54 centimeters 1 yard = 0.9444 meters Metric Units The basic unit of length in the metric system in the meter and is represented by a lowercase m. Standard: The distance traveled by light in absolute vacuum in 1∕299,792,458 of a second. Metric Units 1 Kilometer (km) = 1000 meters 1 Meter = 100 Centimeters (cm) 1 Meter = 1000 Millimeters (mm) Which is larger? A. 1 meter or 105 centimeters C. 12 centimeters or 102 millimeters B. 4 kilometers or 4400 meters D. 1200 millimeters or 1 meter Measuring Length How many millimeters are in 1 centimeter? 1 centimeter = 10 millimeters What is the length of the line in centimeters? _______cm What is the length of the line in millimeters? _______mm What is the length of the line to the nearest centimeter? ________cm HINT: Round to the nearest centimeter – no decimals. -

Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis
Energy & Society Energy Units and Fundamentals Toolkit 1: Energy Units and Fundamentals of Quantitative Analysis 1 Energy & Society Energy Units and Fundamentals Table of Contents 1. Key Concepts: Force, Work, Energy & Power 3 2. Orders of Magnitude & Scientific Notation 6 2.1. Orders of Magnitude 6 2.2. Scientific Notation 7 2.3. Rules for Calculations 7 2.3.1. Multiplication 8 2.3.2. Division 8 2.3.3. Exponentiation 8 2.3.4. Square Root 8 2.3.5. Addition & Subtraction 9 3. Linear versus Exponential Growth 10 3.1. Linear Growth 10 3.2. Exponential Growth 11 4. Uncertainty & Significant Figures 14 4.1. Uncertainty 14 4.2. Significant Figures 15 4.3. Exact Numbers 15 4.4. Identifying Significant Figures 16 4.5. Rules for Calculations 17 4.5.1. Addition & Subtraction 17 4.5.2. Multiplication, Division & Exponentiation 18 5. Unit Analysis 19 5.1. Commonly Used Energy & Non-energy Units 20 5.2. Form & Function 21 6. Sample Problems 22 6.1. Scientific Notation 22 6.2. Linear & Exponential Growth 22 6.3. Significant Figures 23 6.4. Unit Conversions 23 7. Answers to Sample Problems 24 7.1. Scientific Notation 24 7.2. Linear & Exponential Growth 24 7.3. Significant Figures 24 7.4. Unit Conversions 26 8. References 27 2 Energy & Society Energy Units and Fundamentals 1. KEY CONCEPTS: FORCE, WORK, ENERGY & POWER Among the most important fundamentals to be mastered when studying energy pertain to the differences and inter-relationships among four concepts: force, work, energy, and power. Each of these terms has a technical meaning in addition to popular or colloquial meanings. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -
Metric Conversion Worksheet – Solutions
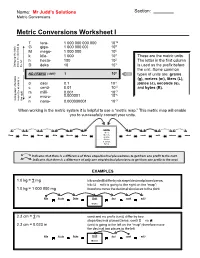
Name: Mr Judd’s Solutions Section: Metric Conversions Metric Conversions Worksheet I T tera- 1 000 000 000 000 1012 9 G giga- 1 000 000 000 10 M mega- 1 000 000 106 k kilo- 1 000 103 These are the metric units. h hecto- 100 102 The letter in the first column 1 Going table up the movethe decimal to theleft D deka 10 10 is used as the prefix before the unit. Some common NO PREFIX ( UNIT) 1 100 types of units are: grams (g), meters (m), liters (L), d deci 0.1 10-1 joules (J), seconds (s), c centi- 0.01 10-2 and bytes (B). m milli- 0.001 10-3 -6 μ micro- 0.000001 10 Goingtable the down movethe decimal to theright n nano- 0.000000001 10-9 When working in the metric system it is helpful to use a “metric map.” This metric map will enable you to successfully convert your units. Units Liters Tera Giga Mega Kilo Hecto Deka Meters deci centi milli micro nano Grams Joules Seconds Bytes indicates that there is a difference of three steps/decimal places/zeros to get from one prefix to the next. indicates that there is a difference of only one step/decimal place/zero to get from one prefix to the next. EXAMPLES 1.0 kg = ? mg 1.0 kg = 1 000 000 mg Kilo Hecto Deka Unit deci centi milli Grams 2.3 cm = ? m 2.3 cm = 0.023 m (unit) is going to the left on the “map”; therefore move the decimal two places to the left Kilo Hecto Deka Unit deci centi milli Meters From To Difference Move decimal in zeros/ left or right? prefix unit prefix unit decimal places/steps km kilo Meters m no prefix Meters 3 right Mg mega grams Gg giga grams 3 left mL milli Litres L no prefix Litres 3 left kJ kilo Joules mJ milli Joules 6 right μs micro seconds ms milli seconds 3 left B no prefix bytes kB kilo bytes 3 left cm centi metre mm milli metre 1 right cL centi Litres μL micro Litres 4 right PROBLEMS 1. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Chapter 1, Section
Chapter 1 Chemical Foundations 1.3 Units of Measurement Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 1 Measurement You make a measurement every time you • measure your height. • read your watch. • take your temperature. • weigh a cantaloupe. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 2 Measurement in Chemistry In chemistry we • measure quantities. • do experiments. • calculate results. • use numbers to report measurements. • compare results to standards. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 3 Measurement In a measurement • a measuring tool is used to compare some dimension of an object to a standard. • of the thickness of the skin fold at the waist, calipers are used. Copyright © 2005 by Pearson Education, Inc. Publishing as Benjamin Cummings 4 Stating a Measurement In every measurement, a number is followed by a unit. Observe the following examples of measurements: Number and Unit 35 m 0.25 L 225 lb 3.4 hr 5 The Metric System (SI) The metric system or SI (international system) is • a decimal system based on 10. • used in most of the world. • used everywhere by scientists. 6 The 7 Basic Fundamental SI Units Physical Quantity Name of Unit Abbreviation Mass kilogram kg Length meter m Time second s Temperature kelvin K Electric current ampere A Amount of substance mole mol Luminous intensity candela cd 7 Units in the Metric System In the metric and SI systems, one unit is used for each type of measurement: Measurement Metric SI Length meter (m) meter (m) Volume liter (L) cubic meter (m3) Mass gram (g) kilogram (kg) Time second (s) second (s) Temperature Celsius (°C) Kelvin (K) 8 Length Measurement Length • is measured using a meter stick. -

How Are Units of Measurement Related to One Another?
UNIT 1 Measurement How are Units of Measurement Related to One Another? I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot express it in numbers, your knowledge is of a meager and unsatisfactory kind... Lord Kelvin (1824-1907), developer of the absolute scale of temperature measurement Engage: Is Your Locker Big Enough for Your Lunch and Your Galoshes? A. Construct a list of ten units of measurement. Explain the numeric relationship among any three of the ten units you have listed. Before Studying this Unit After Studying this Unit Unit 1 Page 1 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. High School Chemistry: An Inquiry Approach 1. Use the measuring instrument provided to you by your teacher to measure your locker (or other rectangular three-dimensional object, if assigned) in meters. Table 1: Locker Measurements Measurement (in meters) Uncertainty in Measurement (in meters) Width Height Depth (optional) Area of Locker Door or Volume of Locker Show Your Work! Pool class data as instructed by your teacher. Table 2: Class Data Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Width Height Depth Area of Locker Door or Volume of Locker Unit 1 Page 2 Copyright © 2012 Montana Partners This project was largely funded by an ESEA, Title II Part B Mathematics and Science Partnership grant through the Montana Office of Public Instruction. -

Measuring in Metric Units BEFORE Now WHY? You Used Metric Units
Measuring in Metric Units BEFORE Now WHY? You used metric units. You’ll measure and estimate So you can estimate the mass using metric units. of a bike, as in Ex. 20. Themetric system is a decimal system of measurement. The metric Word Watch system has units for length, mass, and capacity. metric system, p. 80 Length Themeter (m) is the basic unit of length in the metric system. length: meter, millimeter, centimeter, kilometer, Three other metric units of length are themillimeter (mm) , p. 80 centimeter (cm) , andkilometer (km) . mass: gram, milligram, kilogram, p. 81 You can use the following benchmarks to estimate length. capacity: liter, milliliter, kiloliter, p. 82 1 millimeter 1 centimeter 1 meter thickness of width of a large height of the a dime paper clip back of a chair 1 kilometer combined length of 9 football fields EXAMPLE 1 Using Metric Units of Length Estimate the length of the bandage by imagining paper clips laid next to it. Then measure the bandage with a metric ruler to check your estimate. 1 Estimate using paper clips. About 5 large paper clips fit next to the bandage, so it is about 5 centimeters long. ch O at ut! W 2 Measure using a ruler. A typical metric ruler allows you to measure Each centimeter is divided only to the nearest tenth of into tenths, so the bandage cm 12345 a centimeter. is 4.8 centimeters long. 80 Chapter 2 Decimal Operations Mass Mass is the amount of matter that an object has. The gram (g) is the basic metric unit of mass. -

About the Nature of Gravitational Constant and a Rational Metric System
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/303862772 About the Nature of Gravitational Constant and a Rational Metric System. Working Paper · September 2002 DOI: 10.13140/RG.2.1.1992.9205 1 author: Andrew Wutke Thales Group 6 PUBLICATIONS 1 CITATION SEE PROFILE Available from: Andrew Wutke Retrieved on: 04 July 2016 DRAF"T About The Nature of G6pffi Constant and Rational Metric SYstem Andrew Wutke SYdneY Australia email andreww @ optus hame. c om. au 15 september,2002 "We are to adtnit no mare causes of natural things that such as are both true and sufficient to explain their appearance*" INewon] Abstract The gravitational constant G has been a sutrject of interest for more than two centuries. Precise measurements indicate #bqual to 6'673(I0)xl0'r m3i1tg s21 with relative standard uncertainty of 1.5x10-3[U. The need fcrr such constant is discussed. Various s)6tem of units of measure have emerged since Newton, and none of them is both piaaical, and usefid in thesetical rqsearch. The relevance ofa metric system that only Las length and time as base units is analysed and such system proposed. As a rssult much highm clarity of physical quantitie.s is achieved. Grmi$d;onstant and electric constant can be eliminated to become nondimensional, that results in elimination of of the sarre composite unit for both kilogram of mass and Coulomb 9f charge in fuvour physical quantities being m3/s2. The system offers a great advantage to General Relativity where only space-time units can be used. -

MEMS Metrology Metrology What Is a Measurement Measurable
Metrology • What is metrology? – It is the science of weights and measures • Refers primarily to the measurements of length, MEMS Metrology wetight, time, etc. • Mensuration- A branch of applied geometry – It measure the area and volume of solids from Dr. Bruce K. Gale lengths and angles Fundamentals of Micromachining • It also includes other engineering measurements for the establishment of a flat, plane reference surface What is a Measurement Measurable Parameters • A measurement is an act of assigning a • What do we want to • Pressure specific value to a physical variable measure? • Forces • The physical variable becomes the • Length or distance •Stress measured variable •Mass •Strain • Temperature • Measurements provide a basis for • Friction judgements about • Elemental composition • Resistance •Viscosity – Process information • Roughness • Diplacements or – Quality assurance •Depth distortions – Process control • Intensity •Time •etc. Components of a Measuring Measurement Systems and Tools System • Measurement systems are important tools for the quantification of the physical variable • Measurement systems extend the abilities of the human senses, while they can detect and recognize different degrees of physical variables • For scientific and engineering measurement, the selection of equipment, techniques and interpretation of the measured data are important How Important are Importance of Metrology Measurements? • In human relationships, things must be • Measurement is the language of science counted and measured • It helps us -

SI and CGS Units in Electromagnetism
SI and CGS Units in Electromagnetism Jim Napolitano January 7, 2010 These notes are meant to accompany the course Electromagnetic Theory for the Spring 2010 term at RPI. The course will use CGS units, as does our textbook Classical Electro- dynamics, 2nd Ed. by Hans Ohanian. Up to this point, however, most students have used the International System of Units (SI, also known as MKSA) for mechanics, electricity and magnetism. (I believe it is easy to argue that CGS is more appropriate for teaching elec- tromagnetism to physics students.) These notes are meant to smooth the transition, and to augment the discussion in Appendix 2 of your textbook. The base units1 for mechanics in SI are the meter, kilogram, and second (i.e. \MKS") whereas in CGS they are the centimeter, gram, and second. Conversion between these base units and all the derived units are quite simply given by an appropriate power of 10. For electromagnetism, SI adds a new base unit, the Ampere (\A"). This leads to a world of complications when converting between SI and CGS. Many of these complications are philosophical, but this note does not discuss such issues. For a good, if a bit flippant, on- line discussion see http://info.ee.surrey.ac.uk/Workshop/advice/coils/unit systems/; for a more scholarly article, see \On Electric and Magnetic Units and Dimensions", by R. T. Birge, The American Physics Teacher, 2(1934)41. Electromagnetism: A Preview Electricity and magnetism are not separate phenomena. They are different manifestations of the same phenomenon, called electromagnetism. One requires the application of special relativity to see how electricity and magnetism are united.