Conceptualizing Energy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Technology
Nuclear Technology Joseph A. Angelo, Jr. GREENWOOD PRESS NUCLEAR TECHNOLOGY Sourcebooks in Modern Technology Space Technology Joseph A. Angelo, Jr. Sourcebooks in Modern Technology Nuclear Technology Joseph A. Angelo, Jr. GREENWOOD PRESS Westport, Connecticut • London Library of Congress Cataloging-in-Publication Data Angelo, Joseph A. Nuclear technology / Joseph A. Angelo, Jr. p. cm.—(Sourcebooks in modern technology) Includes index. ISBN 1–57356–336–6 (alk. paper) 1. Nuclear engineering. I. Title. II. Series. TK9145.A55 2004 621.48—dc22 2004011238 British Library Cataloguing in Publication Data is available. Copyright © 2004 by Joseph A. Angelo, Jr. All rights reserved. No portion of this book may be reproduced, by any process or technique, without the express written consent of the publisher. Library of Congress Catalog Card Number: 2004011238 ISBN: 1–57356–336–6 First published in 2004 Greenwood Press, 88 Post Road West, Westport, CT 06881 An imprint of Greenwood Publishing Group, Inc. www.greenwood.com Printed in the United States of America The paper used in this book complies with the Permanent Paper Standard issued by the National Information Standards Organization (Z39.48–1984). 10987654321 To my wife, Joan—a wonderful companion and soul mate Contents Preface ix Chapter 1. History of Nuclear Technology and Science 1 Chapter 2. Chronology of Nuclear Technology 65 Chapter 3. Profiles of Nuclear Technology Pioneers, Visionaries, and Advocates 95 Chapter 4. How Nuclear Technology Works 155 Chapter 5. Impact 315 Chapter 6. Issues 375 Chapter 7. The Future of Nuclear Technology 443 Chapter 8. Glossary of Terms Used in Nuclear Technology 485 Chapter 9. Associations 539 Chapter 10. -

Geoffrey Wilkinson
THE LONG SEARCH FOR STABLE TRANSITION METAL ALKYLS Nobel Lecture, December 11, 1973 by G EOFFREY W ILKINSON Imperial College of Science & Technology, London, England Chemical compounds in which there is a single bond between a saturated car- bon atom and a transition metal atom are of unusual importance. Quite aside from the significance and role in Nature of the cobalt to carbon bonds in the vitamin B 12 system and possible metal to carbon bonds in other biological systems, we need only consider that during the time taken to deliver this lec- ture, many thousands, if not tens of thousands of tons of chemical compounds are being transformed or synthesised industrially in processes which at some stage involve a transition metal to carbon bond. The nonchemist will pro- bably be most familiar with polyethylene or polypropylene in the form of do- mestic utensils, packaging materials, children’s toys and so on. These materials are made by Ziegler-Natta* or Philipps’ catalysis using titanium and chro- mium respectively. However, transition metal compounds are used as catalysts in the synthesis of synthetic rubbers and other polymers, and of a variety of simple compounds used as industrial solvents or intermediates. For example alcohols are made from olefins, carbon monoxide and hydrogen by use of cobalt or rhodium catalysts, acetic acid is made by carbonylation of methanol using rhodium catalysts and acrylonitrile is dimerised to adiponitrile (for nylon) by nickel catalysts. We should also not forget that the huge quantities of petroleum hydrocarbons processed by the oil and petrochemical industry are re-formed over platinum, platinum-rhenium or platinum-germanium sup- ported on alumina. -
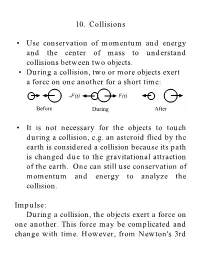
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

James Clerk Maxwell
James Clerk Maxwell JAMES CLERK MAXWELL Perspectives on his Life and Work Edited by raymond flood mark mccartney and andrew whitaker 3 3 Great Clarendon Street, Oxford, OX2 6DP, United Kingdom Oxford University Press is a department of the University of Oxford. It furthers the University’s objective of excellence in research, scholarship, and education by publishing worldwide. Oxford is a registered trade mark of Oxford University Press in the UK and in certain other countries c Oxford University Press 2014 The moral rights of the authors have been asserted First Edition published in 2014 Impression: 1 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission in writing of Oxford University Press, or as expressly permitted by law, by licence or under terms agreed with the appropriate reprographics rights organization. Enquiries concerning reproduction outside the scope of the above should be sent to the Rights Department, Oxford University Press, at the address above You must not circulate this work in any other form and you must impose this same condition on any acquirer Published in the United States of America by Oxford University Press 198 Madison Avenue, New York, NY 10016, United States of America British Library Cataloguing in Publication Data Data available Library of Congress Control Number: 2013942195 ISBN 978–0–19–966437–5 Printed and bound by CPI Group (UK) Ltd, Croydon, CR0 4YY Links to third party websites are provided by Oxford in good faith and for information only. -

Great Physicists
Great Physicists Great Physicists The Life and Times of Leading Physicists from Galileo to Hawking William H. Cropper 1 2001 1 Oxford New York Athens Auckland Bangkok Bogota´ Buenos Aires Cape Town Chennai Dar es Salaam Delhi Florence HongKong Istanbul Karachi Kolkata Kuala Lumpur Madrid Melbourne Mexico City Mumbai Nairobi Paris Sao Paulo Shanghai Singapore Taipei Tokyo Toronto Warsaw and associated companies in Berlin Ibadan Copyright ᭧ 2001 by Oxford University Press, Inc. Published by Oxford University Press, Inc. 198 Madison Avenue, New York, New York 10016 Oxford is a registered trademark of Oxford University Press All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of Oxford University Press. Library of Congress Cataloging-in-Publication Data Cropper, William H. Great Physicists: the life and times of leadingphysicists from Galileo to Hawking/ William H. Cropper. p. cm Includes bibliographical references and index. ISBN 0–19–513748–5 1. Physicists—Biography. I. Title. QC15 .C76 2001 530'.092'2—dc21 [B] 2001021611 987654321 Printed in the United States of America on acid-free paper Contents Preface ix Acknowledgments xi I. Mechanics Historical Synopsis 3 1. How the Heavens Go 5 Galileo Galilei 2. A Man Obsessed 18 Isaac Newton II. Thermodynamics Historical Synopsis 41 3. A Tale of Two Revolutions 43 Sadi Carnot 4. On the Dark Side 51 Robert Mayer 5. A Holy Undertaking59 James Joule 6. Unities and a Unifier 71 Hermann Helmholtz 7. The Scientist as Virtuoso 78 William Thomson 8. -

Thermodynamic Physics and the Poetry and Prose of Gerard Manley Hopkins
Georgia State University ScholarWorks @ Georgia State University English Dissertations Department of English 5-11-2015 Literatures of Stress: Thermodynamic Physics and the Poetry and Prose of Gerard Manley Hopkins Thomas Mapes Follow this and additional works at: https://scholarworks.gsu.edu/english_diss Recommended Citation Mapes, Thomas, "Literatures of Stress: Thermodynamic Physics and the Poetry and Prose of Gerard Manley Hopkins." Dissertation, Georgia State University, 2015. https://scholarworks.gsu.edu/english_diss/134 This Dissertation is brought to you for free and open access by the Department of English at ScholarWorks @ Georgia State University. It has been accepted for inclusion in English Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. LITERATURES OF STRESS: THERMODYNAMIC PHYSICS AND THE POETRY AND PROSE OF GERARD MANLEY HOPKINS by THOMAS MAPES Under the Direction of Paul Schmidt, PhD ABSTRACT This dissertation examines two of the various literatures of energy in Victorian Britain: the scientific literature of the North British school of energy physics, and the poetic and prose literature of Gerard Manley Hopkins. As an interdisciplinary effort, it is intended for several audiences. For readers interested in science history, it offers a history of two terms – stress and strain – central to modern physics. As well, in discussing the ideas of various scientific authors (primarily William John Macquorn Rankine, William Thomson, P.G. Tait, and James Clerk Maxwell), it indicates several contributions these figures made to larger culture. For readers of Hopkins’ poems and prose, this dissertation corresponds with a recent trend in criticism in its estimation of Hopkins as a scientifically informed writer, at least in his years post-Stonyhurst. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

The Avogadro Constant to Be Equal to Exactly 6.02214X×10 23 When It Is Expressed in the Unit Mol −1
[august, 2011] redefinition of the mole and the new system of units zoltan mester It is as easy to count atomies as to resolve the propositions of a lover.. As You Like It William Shakespeare 1564-1616 Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, German Empire, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States and Venezuela 1875 -May 20 1875, BIPM, CGPM and the CIPM was established, and a three- dimensional mechanical unit system was setup with the base units metre, kilogram, and second. -1901 Giorgi showed that it is possible to combine the mechanical units of this metre–kilogram–second system with the practical electric units to form a single coherent four-dimensional system -In 1921 Consultative Committee for Electricity (CCE, now CCEM) -by the 7th CGPM in 1927. The CCE to proposed, in 1939, the adoption of a four-dimensional system based on the metre, kilogram, second, and ampere, the MKSA system, a proposal approved by the ClPM in 1946. -In 1954, the 10th CGPM, the introduction of the ampere, the kelvin and the candela as base units -in 1960, 11th CGPM gave the name International System of Units, with the abbreviation SI. -in 1970, the 14 th CGMP introduced mole as a unit of amount of substance to the SI 1960 Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Much improved atomic weight estimates, oxygen = 100 . Jöns Jacob Berzelius (1779–1848) Further improved atomic weight estimates . -

Page 1 of 29 Dalton Transactions
Dalton Transactions Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/dalton Page 1 of 29 Dalton Transactions Core-level photoemission spectra of Mo 0.3 Cu 0.7 Sr 2ErCu 2Oy, a superconducting perovskite derivative. Unconventional structure/property relations a, b b b a Sourav Marik , Christine Labrugere , O.Toulemonde , Emilio Morán , M. A. Alario- Manuscript Franco a,* aDpto. Química Inorgánica, Facultad de CC.Químicas, Universidad Complutense de Madrid, 28040- Madrid (Spain) bCNRS, Université de Bordeaux, ICMCB, 87 avenue du Dr. A. Schweitzer, Pessac, F-33608, France -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

2019 Redefinition of SI Base Units
2019 redefinition of SI base units A redefinition of SI base units is scheduled to come into force on 20 May 2019.[1][2] The kilogram, ampere, kelvin, and mole will then be defined by setting exact numerical values for the Planck constant (h), the elementary electric charge (e), the Boltzmann constant (k), and the Avogadro constant (NA), respectively. The metre and candela are already defined by physical constants, subject to correction to their present definitions. The new definitions aim to improve the SI without changing the size of any units, thus ensuring continuity with existing measurements.[3][4] In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes,[5][6] which the International Committee for Weights and Measures (CIPM) had proposed earlier that year.[7]:23 The previous major change of the metric system was in 1960 when the International System of Units (SI) was formally published. The SI is a coherent system structured around seven base units whose definitions are unconstrained by that of any other unit and another twenty-two named units derived from these base units. The metre was redefined in terms of the wavelength of a spectral line of a The SI system after the 2019 redefinition: krypton-86 radiation,[Note 1] making it derivable from universal natural Dependence of base unit definitions onphysical constants with fixed numerical values and on other phenomena, but the kilogram remained defined in terms of a physical prototype, base units. leaving it the only artefact upon which the SI unit definitions depend. The metric system was originally conceived as a system of measurement that was derivable from unchanging phenomena,[8] but practical limitations necessitated the use of artefacts (the prototype metre and prototype kilogram) when the metric system was first introduced in France in 1799. -

Streamlining Alloy Design and Thermo-Mechanical Processing Parameters for High Strength Line Pipe Steels and Hot Rolled Microall
University of Texas at El Paso DigitalCommons@UTEP Open Access Theses & Dissertations 2018-01-01 Streamlining Alloy Design And Thermo- Mechanical Processing Parameters For High Strength Line Pipe Steels And Hot Rolled Microalloyed Steels: Process - Structure - Property Paradigm Venkata Vignesh Natarajan University of Texas at El Paso, [email protected] Follow this and additional works at: https://digitalcommons.utep.edu/open_etd Part of the Materials Science and Engineering Commons, and the Mechanics of Materials Commons Recommended Citation Natarajan, Venkata Vignesh, "Streamlining Alloy Design And Thermo-Mechanical Processing Parameters For High Strength Line Pipe Steels And Hot Rolled Microalloyed Steels: Process - Structure - Property Paradigm" (2018). Open Access Theses & Dissertations. 129. https://digitalcommons.utep.edu/open_etd/129 This is brought to you for free and open access by DigitalCommons@UTEP. It has been accepted for inclusion in Open Access Theses & Dissertations by an authorized administrator of DigitalCommons@UTEP. For more information, please contact [email protected]. STREAMLINING ALLOY DESIGN AND THERMO-MECHANICAL PROCESSING PARAMETERS FOR HIGH STRENGTH LINE PIPE STEELS AND HOT ROLLED MICROALLOYED STEELS: PROCESS – STRUCTURE – PROPERTY PARADIGM VENKATA VIGNESH NATARAJAN Doctoral Program in Materials Science and Engineering APPROVED: Devesh Misra, Ph.D., Chair Srinivasa Rao Singamaneni, Ph.D. Guikuan Yue, Ph.D. Charles Ambler, Ph.D. Dean of the Graduate School Copyright © by Venkata Vignesh Natarajan 2018 DEDICATION