Toxicological Profile for Vanadium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vanadium Pentoxide and Other Inorganic Vanadium Compounds
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization. Concise International Chemical Assessment Document 29 VANADIUM PENTOXIDE AND OTHER INORGANIC VANADIUM COMPOUNDS Note that the layout and pagination of this pdf file are not identical to the printed CICAD First draft prepared by Dr M. Costigan and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Centre for Ecology and Hydrology, Huntingdon, United Kingdom Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 2001 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management -

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -

Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and in Vitro Cytotoxicity and Insulin- Like Activity
inorganics Article Kinetic Studies of Sodium and Metforminium Decavanadates Decomposition and In Vitro Cytotoxicity and Insulin- Like Activity Aniela M. Silva-Nolasco 1,2, Luz Camacho 2 , Rafael Omar Saavedra-Díaz 1, Oswaldo Hernández-Abreu 1 , Ignacio E. León 3 and Irma Sánchez-Lombardo 1,* 1 Centro de Investigación de Ciencia y Tecnología Aplicada de Tabasco, División Académica de Ciencias Básicas (CICTAT), Universidad Juárez Autónoma de Tabasco, Carretera Cunduacán-Jalpa km. 1 Col. La Esmeralda, Cunduacán 86690, Tabasco, Mexico; [email protected] (A.M.S.-N.); [email protected] (R.O.S.-D.); [email protected] (O.H.-A.) 2 Laboratorio de Nutrición Experimental, Instituto Nacional de Pediatría, Ciudad de Mexico 04530, Mexico; [email protected] 3 Centro de Química Inorgánica CEQUINOR (CONICET, UNLP), Bv 120 1465, La Plata 1900, Argentina; [email protected] * Correspondence: [email protected] Received: 22 October 2020; Accepted: 2 December 2020; Published: 8 December 2020 Abstract: The kinetics of the decomposition of 0.5 and 1.0 mM sodium decavanadate (NaDeca) and metforminium decavanadate (MetfDeca) solutions were studied by 51V NMR in Dulbecco’s modified Eagle’s medium (DMEM) medium (pH 7.4) at 25 ◦C. The results showed that decomposition products 2 4 are orthovanadate [H2VO4]− (V1) and metavanadate species like [H2V2O7] − (V2), [V4O12] − (V4) 5 and [V5O15] − (V5) for both compounds. The calculated half-life times of the decomposition reaction were 9 and 11 h for NaDeca and MetfDeca, respectively, at 1 mM concentration. The hydrolysis products that presented the highest rate constants were V1 and V4 for both compounds. -
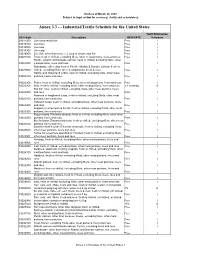
US Schedule for Internet V2
Draft as of March 23, 2007 Subject to legal review for accuracy, clarity and consistency. Annex 3.3 - - Industrial/Textile Schedule for the United States Tariff Elimination US 8 digit Description MFN RATE Schedule 03011000 Live ornamental fish Free I 03019100 Live trout Free I 03019200 Live eels Free I 03019300 Live carp Free I 03019900 Live fish, other than trout, eel, carp or ornamental fish Free I 03021100 Trout, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I Pacific, Atlantic and Danube salmon, fresh or chilled, excluding fillets, other 03021200 meat portions, livers and roes Free I Salmonidae other than trout or Pacific, Atlantic & Danube salmon, fresh or 03021900 chilled, excluding fillets, other meat portions, livers & roes Free I Halibut and Greenland turbot, fresh or chilled, excluding fillets, other meat 03022100 portions, livers and roes Free I 03022200 Plaice, fresh or chilled, excluding fillets, other meat portions, livers and roes Free I 03022300 Sole, fresh or chilled, excluding fillets, other meat portions, livers and roes 1.1 cents/kg A Flat fish, nesi, fresh or chilled, excluding fillets, other meat portions, livers 03022900 and roes Free I Albacore or longfinned tunas, fresh or chilled, excluding fillets, other meat 03023100 portions, livers and roes Free I Yellowfin tunas, fresh or chilled, excluding fillets, other meat portions, livers 03023200 and roes Free I Skipjack or stripe-bellied bonito, fresh or chilled, excluding fillets, other meat 03023300 portions, livers and roes Free -

Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life
Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life Item Type text; Electronic Dissertation Authors Pasek, Matthew Adam Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 07/10/2021 06:16:37 Link to Item http://hdl.handle.net/10150/194288 PHOSPHORUS AND SULFUR COSMOCHEMISTRY: IMPLICATIONS FOR THE ORIGINS OF LIFE by Matthew Adam Pasek ________________________ A Dissertation Submitted to the Faculty of the DEPARTMENT OF PLANETARY SCIENCE In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College UNIVERSITY OF ARIZONA 2 0 0 6 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Matthew Adam Pasek entitled Phosphorus and Sulfur Cosmochemistry: Implications for the Origins of Life and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 04/11/2006 Dante Lauretta _______________________________________________________________________ Date: 04/11/2006 Timothy Swindle _______________________________________________________________________ Date: 04/11/2006 -

PROVISIONAL PEER-REVIEWED TOXICITY VALUES for VANADIUM and ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and Others)
EPA/690/R-09/070F l Final 9-30-2009 Provisional Peer-Reviewed Toxicity Values for Vanadium and Its Soluble Inorganic Compounds Other Than Vanadium Pentoxide (CASRN 7440-62-2 and Others) Derivation of Subchronic and Chronic Oral RfDs Superfund Health Risk Technical Support Center National Center for Environmental Assessment Office of Research and Development U.S. Environmental Protection Agency Cincinnati, OH 45268 Commonly Used Abbreviations BMD Benchmark Dose IRIS Integrated Risk Information System IUR inhalation unit risk LOAEL lowest-observed-adverse-effect level LOAELADJ LOAEL adjusted to continuous exposure duration LOAELHEC LOAEL adjusted for dosimetric differences across species to a human NOAEL no-observed-adverse-effect level NOAELADJ NOAEL adjusted to continuous exposure duration NOAELHEC NOAEL adjusted for dosimetric differences across species to a human NOEL no-observed-effect level OSF oral slope factor p-IUR provisional inhalation unit risk p-OSF provisional oral slope factor p-RfC provisional inhalation reference concentration p-RfD provisional oral reference dose RfC inhalation reference concentration RfD oral reference dose UF uncertainty factor UFA animal to human uncertainty factor UFC composite uncertainty factor UFD incomplete to complete database uncertainty factor UFH interhuman uncertainty factor UFL LOAEL to NOAEL uncertainty factor UFS subchronic to chronic uncertainty factor i FINAL 9-30-2009 PROVISIONAL PEER-REVIEWED TOXICITY VALUES FOR VANADIUM AND ITS SOLUBLE INORGANIC COMPOUNDS OTHER THAN VANADIUM PENTOXIDE (CASRN 7440-62-2 and others) Background On December 5, 2003, the U.S. Environmental Protection Agency's (U.S. EPA) Office of Superfund Remediation and Technology Innovation (OSRTI) revised its hierarchy of human health toxicity values for Superfund risk assessments, establishing the following three tiers as the new hierarchy: 1) U.S. -

Vanadate-Molybdate Reagent Safety Data Sheet According to Federal Register / Vol
Vanadate-Molybdate Reagent Safety Data Sheet according to Federal Register / Vol. 77, No. 58 / Monday, March 26, 2012 / Rules and Regulations Date of issue: 12/20/2013 Revision date: 05/02/2014 Supersedes: 12/20/2013 Version: 1.1 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1. Product identifier Product form : Mixture Product name : Vanadate-Molybdate Reagent Product code : LC26600 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : For laboratory and manufacturing use only. 1.3. Details of the supplier of the safety data sheet LabChem Inc Jackson's Pointe Commerce Park Building 1000, 1010 Jackson's Pointe Court Zelienople, PA 16063 - USA T 412-826-5230 - F 724-473-0647 [email protected] - www.labchem.com 1.4. Emergency telephone number Emergency number : CHEMTREC: 1-800-424-9300 or 011-703-527-3887 SECTION 2: Hazards identification 2.1. Classification of the substance or mixture GHS-US classification Skin Corr. 1B H314 Eye Dam. 1 H318 2.2. Label elements GHS-US labelling Hazard pictograms (GHS-US) : GHS05 Signal word (GHS-US) : Danger Hazard statements (GHS-US) : H314 - Causes severe skin burns and eye damage Precautionary statements (GHS-US) : P260 - Do not breathe mist, vapours, spray P264 - Wash exposed skin thoroughly after handling P280 - Wear protective gloves, eye protection, protective clothing, face protection P301+P330+P331 - IF SWALLOWED: rinse mouth. Do NOT induce vomiting P303+P361+P353 - IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower P304+P340 - IF INHALED: remove victim to fresh air and keep at rest in a position comfortable for breathing P305+P351+P338 - If in eyes: Rinse cautiously with water for several minutes. -

Gravimetric Determination of Vanadium As V(IV)-Oxinate
Chem. Anal. (Warsaw), 38, 639 (1993) Gravimetric Determination of Vanadium as V(IV)-Oxinate by S. Kaur, A. K. Chhakkarand L. R. Kakkar* Department ofChemistry, Kurukshetra University, Kurukshetra -132119, Haryana; India Key words: vanadium(V), oxine, 8-hydroxyquinoline, gravimetry A very simple gravimetric method for the determination of vanadium has been worked out. In acid medium vanadium is reduced to tetravalent state which forms greenish-black precipitate with 8-hydroxyquinoline (2 % in 2 mol 1-1 CH3COOH) in the presence of ammonium acetate. The precipitate is dried and weighedas VO(C9H60 N)2. The con version factor for vanadium is 0.1437. The method is free from the interference of molybdenum(VI), chromium(III,VI), uranium(VI), selenium(IV), arsenic(I1I), bis muth(III), lead(U), calcium(II), manganese(I1) and maguesium(II). Opracowanoprosta wagow'\ metode oznaczania wanadu. Wauad(V) redukuje silt w kwasnym roztworze do wanadu(IV), ktory w obecnosci octanuamonu tworzy z 8-hydro ksychinolina (2 % roztwor w 2 mol 1-1CH3COOH) zielonoczaruy osad. Po wysuszeniu osad jestwazonyjako VO(C9H60 Nh Mnoznik analityczny wynosi dla wanadu 0,1437. W oznaczeniu nie przeszkadzaja: molibden(VI), chrom(III,VI), uran(VI), selen(IV), arsen(III), bizmut(III), o16w(II), wapriffl), mangan(II} i magnez(II), Many inorganic [1-4a] and organic [5-8, 11] precipitants employed for the estimation of vanadium in milligram amounts are. unsuitable for routine analysis, either because they are not quantitative or because other elements are coprecipitated withvanadium, and also in some cases, the precipitate formed does not have a definite composition. Cup ferron [4b] is generally recommended for the precipitation of vanadium, but several precautions are necessary because ofthe instability of cupfer ron and many other elements are precipitated by the reagent under conditions ofthe * Senior author for correspondence. -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12 -

Environmental Protection Agency § 712.30
Environmental Protection Agency § 712.30 (2) A confidentiality statement § 712.30 Chemical lists and reporting signed and dated by an authorized per- periods. son located at the plant site or cor- (a)(1) Persons subject to this subpart porate headquarters of the respondent B must submit a Preliminary Assess- company. ment Information Manufacturer’s Re- (3) The specific chemical name and port for each chemical substance or Chemical Abstracts Service (CAS) Reg- mixture that is listed or designated in istry Number listed in 40 CFR 712.30. this section. (4) The name, company, address, city, State, ZIP code, and telephone number (2) Unless a respondent has already of a person who is submitting the form, prepared a Manufacturer’s Report in which may be a person located at a conformity with conditions set forth in plant site or corporate headquarters paragraph (a)(3) of this section, the in- that will serve as the respondent, and formation in each Manufacturer’s Re- will be able to answer questions about port must cover the respondent’s latest the information submitted by the com- complete corporate fiscal year as of the pany to EPA. A respondent to this sub- effective date. The effective date will part must include the appropriate Dun be 30 days after the FEDERAL REGISTER and Bradstreet Number for each plant publishes a rule amendment making site reported. the substance or mixture subject to (5) The plant site activities, such as this subpart B. the manufacturing of a chemical sub- (3) Persons subject to this subpart B stance, including the total quantity of need not comply with the requirements the chemical substance (in kilograms) of paragraph (a)(2) of this section if imported in bulk during the reporting they meet either one of the following period. -

Chemical Analyses for Selected Minor Elements in Pierre Shale
Chemical Analyses for Selected Minor Elements in Pierre Shale GEOLOGICAL SURVEY PROFESSIONAL PAPER 391-A Chemical Analyses for Selected Minor Elements in Pierre Shale By L. F. RADER and F. S. GRIMALDI ANALYTICAL METHODS IN GEOCHEMICAL INVESTIGATIONS OF THE PIERRE SHALE GEOLOGICAL SURVEY PROFESSIONAL PAPER 391-A UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1961 UNITED STATES DEPARTMENT OF THE INTERIOR STEW ART L. UDALL, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U.S. Government Printing Office Washington 25, D.C. CONTENTS Page Page Abstract--_________________________________________ A-l Methods for the determination of individual Introduction ___-----_-_--_________________-__--__-_ 1 constituents Continued General remarks._________________________ ______ 1 Copper lead, and zinc Continued Laboratories. -._---____-_-______________________ 2 Zinc, dithizone method._____^_______________ A-20 Acknowledgments. ___-_-_-_--______--_-_________ 2 Lead, dithizone method______------_-____-__- 22 Division of work________________________________ 2 Arsenic.___________,__________-_-_--___-__---_- 23 Methods selected and treatment of data ___________ 3 Acid digestion, heteropoly blue method________ 23 Sample preparation _________________________________ 4 Alternative fusion-heteropoly blue method_____ 24 Methods for the determination of individual constituents. 4 Titanium. _____________________________________ 4 Selenium, distillation, visual-estimation method.____ 25 Peroxide method._--_--_-____._____________ 4 Molybdenum and tungsten______-____------_---_- 27 Alternative tiron method, ___________________ 5 Isolation by method 1, alpha-benzoinoxime pre Vanadium. _--.---_____-_-___.__________________ 7 cipitation. ___----_-_-_-__--_----_-----_-- 27 Fusion-leach separation method. _____________ 7 Isolation by alternative method 2, alpha-ben Alternative cupferron separation method...... -

Aluminum-Vanadium System Donald Joseph Kenney Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1953 Aluminum-vanadium system Donald Joseph Kenney Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Physical Chemistry Commons Recommended Citation Kenney, Donald Joseph, "Aluminum-vanadium system " (1953). Retrospective Theses and Dissertations. 13302. https://lib.dr.iastate.edu/rtd/13302 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI mmMm-immim STSTEM BmaM S, Ktrmey A Mssertattioa aibaitted to the Sradttate Paeulty in fsKptial Fulfillffleat of the l®cpiir«a®ats tor the Degree of eocfoa or piiLosoFif Sabjecti aysiefil CheBdstry ^proved f Signature was redacted for privacy. In C2iarg®;!6f Ifejor Work Signature was redacted for privacy. Signature was redacted for privacy. Iowa State College 1953 UMI Number: DP12420 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion.