Study on Distribution and Behavior of PFOS (Perfluorooctane Title Sulfonate) and PFOA (Perfluorooctanoate) in Water Environment( Dissertation 全文 )
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The History, Tradition, and Culture of Kyoto Prefecture
The History, Tradition, and Culture of Kyoto Prefecture Kyoto Prefectural Education Center Preface The world has become a smaller place due to the development of high-speed machines and information technology. Nowadays the ability to show the world our identity as Japanese people including our culture and tradition, especially the ability to communicate with the rest of the world, is needed more than ever. Throughout history, Kyoto has been at the center of Japanese culture and history. Kyoto is therefore a good starting point for communicating with the world about Japanese culture and history. In this textbook, the history, tradition, and culture of each region in Kyoto Prefecture are introduced in English so that you, as high school students, can be proud to tell people from abroad about your hometown and Kyoto in English. The contents of chapter Ⅰto Ⅲ in this textbook are based on a Japanese textbook which was written for new teachers working in Kyoto Prefecture. Some parts have been erased and changed, and in other parts, new information was added so that high school students can understand better. You might find some difficult words in the textbook, however, the sentence structures are rather simple and readers with a basic knowledge of grammar can read on with a dictionary at hand. Furthermore, as the Japanese explanations are available on the right page, you can utilize them as a reference if the English is too difficult to understand. Besides, this textbook would be useful not only for students but also for people from abroad who don't know much about Kyoto Prefecture. -

Volki (Annelida: Clitellata: Propappidae) from Japan
JapaneseJapaneseSociety Society ofSystematicof Systematic Zoology Species Diversity, 2006, 11, 359-365 New Record of Propappus volki (Annelida: Clitellata: Propappidae) from Japan Takaaki Torii ll)Et`I CDnsuttants inc., 1334-5Riemon, Oigawa. Shida, Shizuoka, 421-CL212 .lapan E-mail: [email protected] (Received 1 November 2005; Accepted 11 July 2006) A species of freshwater oligochaete, PrQpoppus volki Michaelsen, 1916, is newly recorded from sand and gravel bottoms of several unpolluted streams in Honshu, Japan. The present material agrees well with the previous de- scriptiens of this species except that the spermathecal ampulla is shorter and the proboscis is more elomgate than those described earlier. This is the first record of the family Propappidae Coates, 1986 (New Japanese name: Ko- himemimizu-ka) and the genus Prql)qppus Miehaelsen, 1905 (New Japanese narne: Ko-himemimizu-zoku) in Japan. It provides evidence that the oligochaete fauna in Japan is more closely related to the Holarctic than to the Sino-Indian zoogeographical region, PrQpqppus votici can be regarded as a good bio-indicator fbr unpolluted water. Key Words: oligochaetes, Clitellata, Propappidae, Propappus volki, new record, Japan. Introduction The genus PrQpqppus was erected by Michaelsen (1905) fbr PrQpappus gtandu- losus Michaelsen, 1905 in the family Enchytraeidae. Afterwards Coates (1986) cre- ated the family Propappidae for the single genus PrQpappus on the basis of a unique combination of setal and genital characteristics distinctly different from those ef the Enchytraeidae. Only three species of Propampus have so far been described from freshwater habitats of the Palaearctic region, Among them, Prqpqppus glandulosus has been recorded from Lake Baikal and the Angara and Yenisey Rivers in central Russia (Michaelsen 1905; Coates 1986), PrQpqppus arhynchotus Sokolskaja, 1972 has been recorded only from the Kamchatka Peninsula and Amur basin in the Russian Far East (Timm 1994, 1999a). -

Flood Loss Model Model
GIROJ FloodGIROJ Loss Flood Loss Model Model General Insurance Rating Organization of Japan 2 Overview of Our Flood Loss Model GIROJ flood loss model includes three sub-models. Floods Modelling Estimate the loss using a flood simulation for calculating Riverine flooding*1 flooded areas and flood levels Less frequent (River Flood Engineering Model) and large- scale disasters Estimate the loss using a storm surge flood simulation for Storm surge*2 calculating flooded areas and flood levels (Storm Surge Flood Engineering Model) Estimate the loss using a statistical method for estimating the Ordinarily Other precipitation probability distribution of the number of affected buildings and occurring disasters related events loss ratio (Statistical Flood Model) *1 Floods that occur when water overflows a river bank or a river bank is breached. *2 Floods that occur when water overflows a bank or a bank is breached due to an approaching typhoon or large low-pressure system and a resulting rise in sea level in coastal region. 3 Overview of River Flood Engineering Model 1. Estimate Flooded Areas and Flood Levels Set rainfall data Flood simulation Calculate flooded areas and flood levels 2. Estimate Losses Calculate the loss ratio for each district per town Estimate losses 4 River Flood Engineering Model: Estimate targets Estimate targets are 109 Class A rivers. 【Hokkaido region】 Teshio River, Shokotsu River, Yubetsu River, Tokoro River, 【Hokuriku region】 Abashiri River, Rumoi River, Arakawa River, Agano River, Ishikari River, Shiribetsu River, Shinano -

Geographical Variations in Morphological Characters of the Fluvial Eight-Barbel Loach, Nagare-Hotoke-Dojo (Cobitidae: Nemacheilinae)
Biogeography 17. 43–52. Sep. 20, 2015 Geographical variations in morphological characters of the fluvial eight-barbel loach, Nagare-hotoke-dojo (Cobitidae: Nemacheilinae) Taiki Ito*, Kazuhiro Tanaka and Kazumi Hosoya Program in Environmental Management, Graduate School of Agriculture, Kinki University, 3327-204 Nakamachi, Nara 631-8505, Japan Abstract. The morphological and color variations of Lefua sp. 1 Nagare-hotoke-dojo individuals from 13 river systems were examined. Analysis of variance revealed highly significant variations in Lefua sp. 1 mor- phology and coloration among the 13 populations examined, across all 19 measurements and counts. The 13 populations of Lefua sp. 1 were classified into two major clusters (I and II) by using UPGMA cluster analy- sis. Cluster I comprised fish from the Maruyama, Yura, Muko, Mihara, Yoshino, Hidaka, Kumano, Yoshii, Chikusa, and Ibo river systems. Cluster II comprised fish from the Yoshida, Saita, and Sumoto river systems. Cluster I was further subdivided into sub-clusters: I-i (the Maruyama, Yura, Muko, Mihara, Yoshino, Hidaka, Kumano, and Yoshii river systems) and I-ii (the Chikusa and Ibo river systems). Principal component analysis revealed that populations within cluster II clearly possessed longer caudal peduncles, while populations within cluster I possessed a longer anterior body on average and a deeper body. Populations within sub-cluster I-ii possessed a higher average dorsal fin and a longer average dorsal fin base than those of populations within sub-cluster I-i. A strong correlation was noted between the PC3 score and population latitude (r = 0.621). Observations of body color patterns revealed that individuals from the Yoshino, Mihara, Sumoto, and Hidaka river systems had dark brown mottling on both sides and the dorsal regions of their bodies and many small dark brown spots on the dorsal and caudal fins, while those from the Yura, Muko, and Kumano river systems possessed neither. -

Aomori Cycling
AOMORI CYCLING Up close and personal with the incredible nature and culture of Aomori Aomori Cycling Up close and personal with the incredible nature and culture of Aomori Surrounded by ocean on all sides and blessed by incredible nature such as Shirakami-Sanchi and Lake Towada, each region in Aomori Prefecture enjoys its own unique history, culture and delicacies. Cycling Aomori is the perfect way to discover the intricacies of these individual regions. If you are ready, then jump on your bike and let's head off to explore the beauty of Aomori! 9 1 Course 1 NATSUDOMARI 11 2 Course 2 TOWADA・OIRASE 13 3 Course 3 HIROSAKI 15 4 Course 4 NISHIKAIGAN 17 5 Course 5 OKU-TSUGARU AOMORI 19 6 Course 6 SHIMOKITA 21 7 Course 7 LAKE OGAWARA CYCLING 23 Course 8 HACHINOHE Up close and personal with the incredible nature and culture of Aomori 8 25 33 27 35 29 37 31 7 8 03 02 04 05 06 01/The masses of blue net stored at the port are shellfish baskets, used for cultivating scallops. It's like riding through a maze. 02/Old fishing vessels of all shapes and sizes are dotted around the Natsudomari Peninsula. 03/Showing off the size of the scallops at "Hotate Hiroba," which has a giant scallop as its signboard. You can learn about scallop cultivation on the second floor.04 /At Yogoshiyama Forest Park you can see over 3,000 varieties of succulents. 05/Two men and a cat taking a break from cycling beside the tetrapod seawall. -

Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence Against Panmixia and Support for Sympatric Speciation
G C A T T A C G G C A T genes Article Whole-Genome Sequencing of 84 Japanese Eels Reveals Evidence against Panmixia and Support for Sympatric Speciation Yoji Igarashi 1 , Hong Zhang 1, Engkong Tan 1, Masashi Sekino 2, Kazutoshi Yoshitake 1, Shigeharu Kinoshita 1 , Susumu Mitsuyama 1, Tatsuki Yoshinaga 3, Seinen Chow 2, Hiroaki Kurogi 4, Akira Shinoda 5, Yu-San Han 6, Ryoshiro Wakiya 7, Noritaka Mochioka 7, Toshihiro Yamamoto 4, Hiroshi Kuwada 8,†, Yoshitsugu Kaji 9, Yutaka Suzuki 10, Takashi Gojobori 11, Takanori Kobayashi 2, Kenji Saitoh 2,‡ , Shugo Watabe 3 and Shuichi Asakawa 1,* 1 Department of Aquatic Bioscience, Graduate School of Agricultural and Life Sciences, The University of Tokyo, Bunkyo, Tokyo 113-8657, Japan; [email protected] (Y.I.); [email protected] (H.Z.); [email protected] (E.T.); [email protected] (K.Y.); [email protected] (S.K.); [email protected] (S.M.) 2 National Research Institute of Fisheries Science, Japan Fisheries Research and Education Agency, Yokohama, Kanagawa 236-8648, Japan; [email protected] (M.S.); [email protected] (S.C.); [email protected] (T.K.); [email protected] (K.S.) 3 School of Marine Biosciences, Kitasato University, Sagamihara, Kanagawa 252-0373, Japan; [email protected] (T.Y.); [email protected] (S.W.) 4 Yokosuka Laboratory, National Research Institute of Aquaculture, Japan Fisheries Research and Education Agency, Yokosuka, Kanagawa 238-0316, Japan; [email protected] (H.K.); [email protected] (T.Y.) 5 Department -

Title Freshwater Migration and Feeding Habits of Juvenile Temperate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Kyoto University Research Information Repository Freshwater migration and feeding habits of juvenile temperate Title seabass Lateolabrax japonicus in the stratified Yura River estuary, the Sea of Japan Fuji, Taiki; Kasai, Akihide; Suzuki, Keita W.; Ueno, Masahiro; Author(s) Yamashita, Yoh Citation Fisheries Science (2010), 76(4): 643-652 Issue Date 2010-07 URL http://hdl.handle.net/2433/128758 Right The original publication is available at www.springerlink.com Type Journal Article Textversion author Kyoto University 1 TITLE 2 3 Freshwater migration and feeding habits of juvenile temperate seabass Lateolabrax 4 japonicus in the stratified Yura River estuary, the Sea of Japan 5 Taiki Fuji1*, Akihide Kasai1, Keita W. Suzuki1, Masahiro Ueno2, Yoh Yamashita2 6 7 1Kyoto University, Graduate School of Agriculture, Oiwake, Kitashirakawa, Sakyo, Kyoto 8 606-8502, Japan 9 2Kyoto University, Field Science Education and Research Center, Nagahama, Maizuru 10 625-0086, Japan 11 12 *Corresponding author: Tel:075-606-8502, Fax:075-606-8502 13 Email: [email protected] 14 1 15 ABSTRACT: Juveniles temperate seabass Lateolabrax japonicus were sampled along the 16 Yura River estuary from April to July 2008 to determine their distribution and feeding 17 habits during migration within a microtidal estuary. Juveniles were distributed not only in 18 the surf zone, but also in the freshwater zone and they were particularly abundant 19 associated with aquatic vegetations in the freshwater zone, throughout the sampling period. 20 This distribution pattern suggests that the early life history of the temperate seabass 21 depends more intensively on the river than previously considered. -

Program Book
DVORRUJMDSDQ Association for the Sciences of Limnology and Oceanography Meeting Program ASLO Contents Welcome! ..........................................................................................2 Conference Events ........................................................................12 Meeting Sponsors ...........................................................................2 Public Symposium on Global Warming...........................................12 Meeting Supporters .......................................................................2 Opening Welcome Reception.............................................................12 Organizing Committee .................................................................2 ASLO Membership Business Meeting .............................................12 Poster Sessions and Receptions .........................................................12 Co-Chairs ..................................................................................................2 Scientifi c Committee ..............................................................................2 Workshops and Town Hall Meetings ......................................12 Local Organizing Committee ...............................................................2 L&O e-Lectures Town Hall Meeting ................................................12 Advisory Committee ..............................................................................2 Workshop: Th e Future of Ecosystems Science ...............................12 ASLO Student -

International Symposium on Connectivity of Hills, Humans and Oceans (Cohho) Integrated Ecosystem Management from Hill to Ocean
International Symposium on Connectivity of Hills, Humans and Oceans (CoHHO) Integrated ecosystem management from Hill to Ocean November 26th, 2013 10:00~12:00 Ceremony for 10th Anniversary of FSERC, Kyoto University 12:00~13:00 Lunch 12:30~ Registration for symposium 13:00~13:20 Opening Address by Yoh Yamashita (EUCoHHO, Kyoto Univ.) 13:20~17:00 Oral Session Session1. Connectivity between ecosystems and its disruption (Inamori Hall) Chair person : R. Tateno (FSERC, Kyoto Univ.) 13:20~13:50 Keynote speech by John S. Richardson (Univ. of British Columbia) [Why we need to protect the forest-stream connection to ensure water security and ecosystem services] Chair person : Reiji Masuda (FSERC, Kyoto Univ.) 1-1. Yemi Akegbejo-Samsons (Dept. Agr. Fish. Management, Federal Univ. Agr., Nigeria) : Watershed and Tropical Rain Forest Management Options in Sub-Saharan Africa: Anecdote for Climate Change Mitigation (13:50~14:10) 1-2. Hoan Hai (The University of Danang, Vietnam) and Le Hoang Son (Danang University of Technology) : Eco-Action21 Implementation in Danang City, VietNam (14:10~14:30) 1-3. Khan Md. Golam Mostafa*, Cong-qiang Liu (Institute of Geochemistry, China), 1 Takahito Yoshioka (FSERC, Kyoto Univ., Japan), Marco Minella, Davide Vione (Univ. degli Studi di Torino, Italy) and Daisuke Minakata (Michigan Technological Univ., USA) : Organic Matter of Hills and Humans Origin: Implications to Harmful Algal Blooms, Microbial Food Web, and Ocean Acidification from Source to Seas (14:30~14:50) 1-4. Nicolai Shadrin (Inst. of Biol. of Southern Seas, Ukraine) : A Sea Begins in the Mountains: The Black Sea Cases of the Results from an Altering of Watershed Connectivity by Humans (14:50~15:10) Coffee Break (15:10~15:30) Chair person : J. -

Japan, the Rise of a Modern Power
Ci.T ' ^ '* - \' \ i-:*<^.r •It i\' -"r -.1, -. I'; ft . V ^ , , >^i >. .k ,, \t CORNELL UNIVERSITY LIBRARY THE CHARLES WILLIAM WASON COLLECTION ON CHINA AND THE CHINESE DS SSsAX"""'"'"""'*'"''""^ Japan, the rise of a modern power, 3 1924 012 910 893 Cornell University Library The original of this book is in the Cornell University Library. There are no known copyright restrictions in the United States on the use of the text. http://www.archive.org/details/cu31 92401 291 0893 OUFFIN JAPAN The Rise of a Modern Power Oxford University Press London Edinburgh Glasgow New York Toronto Melbourne Cape Town Bonibay Humphrey Milford Publisher to the University JAPAN The Rise of a Modern Power By ROBERT P. PORTER AUTHOR OF ' THE FULL RECOGNITION OF JAPAN ', ETC OXFORD AT THE CLARENDON PRESS 1918 w/o?7/o PREFACE My father died while this book was in an incomplete state, although the greater part of it was in type ; and it is owing to the kindness of several of his friends who have consented to put the work into final form that it has become possible to publish it. In writing it his object was to describe, for English- speaking people, the main facts of Japanese history. Despite the efforts of numerous Orientalists to popularize knowledge of Japanese history and of Japanese political, economic, and social conditions, he felt that a great deal has yet to be done before the average British youth and adult will be as familiar with Japan as the average Japanese youth and adult are with Great Britain. -

12183018 01.Pdf
あああ プロジェクト業務完了報告書 報告書の構成 ケニア共和国 洪水に脆弱な地域における効果的な洪水管理のための能力開発プロジェクト プロジェクト業務完了報告書 報告書の構成 第1巻 主報告書 第Ⅰ部 プロジェクトの背景と概要 第Ⅱ部 プロジェクト活動内容(成果 1) 第Ⅲ部 プロジェクト活動内容(成果 2) 第Ⅳ部 プロジェクト活動内容(全成果共通) 添付資料 第 2 巻 技術協力成果品 第 3 巻 付属資料(1/2) 第 4 巻 付属資料(2/2) 適用通貨換算率 USD1.00 = Ksh 85.02 = JPY 102.20 円 2014 年 3 月 ケニア共和国洪水に脆弱な地域における 効果的な洪水管理のための能力開発プロジェクト 第 3 巻 付属資料 リスト(1/2) 第1部 プロジェクトの背景と概要 付属資料 1-1 WRMA 職員の洪水管理に係るキャパシティの変化 第2部 プロジェクト活動内容(成果 1) 付属資料 2-1 WRMA 洪水災害データベース(案) 付属資料 2-2 WRMA 職員の能力評価指標 付属資料 2-3 WRUA メンバーの能力評価指標 付属資料 2-4 WDC マニュアル洪水モジュール抜粋版 付属資料 2-5 本邦研修実施報告書 第3部 プロジェクト活動内容(成果 2) 付属資料 3-1 コミュニティベース洪水ハザードマップ作成活動報告書 付属資料 3-2 Isiolo 川流域洪水管理計画案 付属資料 3-3 Lumi 川流域洪水管理計画案 付属資料 3-4 Gucha Migori 川流域洪水管理計画案 第 4 巻 付属資料 リスト(2/2) 付属資料 3-5 Constitution of IFMC 付属資料 3-6 Isiolo 川流域における第 1 回 IFMC 会議議事録等 付属資料 3-7 Isiolo 川流域における第 2 回 IFMC 会議議事録等 付属資料 3-8 Isiolo 川流域における第 3 回 IFMC 会議議事録等 付属資料 3-9 Lumi 川流域における第 1 回 IFMC 会議議事録等 付属資料 3-10 Lumi 川流域における第 2 回 IFMC 会議議事録等 付属資料 3-11 Lumi 川流域における第 3 回 IFMC 会議議事録等 付属資料 3-12 Gucha Migori 川流域における第 1 回 IFMC 会議議事録等 付属資料 3-13 Gucha Migori 川流域における第 2 回 IFMC 会議議事録等 付属資料 3-14 Gucha Migori 川流域における第 3 回 IFMC 会議議事録等 付属資料 3-15 Gucha Migori 川流域における第 4 回 IFMC 会議議事録等 付属資料 3-16 Isiolo コミュニティ防災活動実施計画案 付属資料 3-17 MOU-Procurement-Com-Isiolo 付属資料 3-18 Minutes-Procurement-Com-Isiolo 付属資料 3-19 MOU-Riverbank protection works-Isiolo 付属資料 3-20 Isiolo パイロット事業契約報告 付属資料 3-21 Lumi コミュニティ防災活動実施計画案 付属資料 3-22 MOU-Procurement-Com-Lumi 付属資料 3-23 Minutes-Procurement-Com-Lumi 付属資料 3-24 MOU-Environmental improvement of evacuation camp-Lumi 付属資料 3-25 Lumi パイロット事業契約報告 付属資料 3-26 -
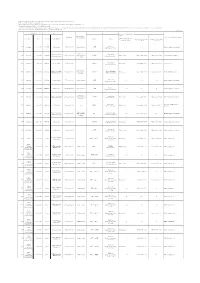
Muscle Part Is Used As a Sample Unless Otherwise Noted
Results of the inspection on radioactive materials in fisheries products (press releases in October,2012䡚December,2012) Muscle part is used as a sample unless otherwise noted. The total value of cesium is written to two significant figures in accordance with the notice issued by the Ministry of Health, Labour and Welfare. The numbers in the parenthesis indicate the detection limit of analysis. Except for the test operations for giant pacific octopus, chesnut octopus,Japanese flying squid, spear squid, horsehair crab, snow crab, whelks (Buccinum isaotakii, Neptunea constricta , Neptunea intersculpta ,and Beringius polynematicus) , thornhead, greeneyes and rikuzen flounder there are no operations of any coastal and trawl fisheries in the sea area off Fukushima Prefecture currently.(half-tone dot cells) 29 March 2013 Press release Origin Item Radioactive Caesium (Bq/kg) Total Name of sampling Sampling date Facility that conducted the analysis area on food labeling Prefecture Date Prefecture Landing port or area Japanese English Standard limit for Radioactive Radioactive Caesium (Bq/kg) Radioactive Caesium (Bq/kg) Cesium in fish: 100Bq/kg Cesium-134 Cesium-137 Pacific cod 9935 Hokkaido 1 October 2012 Hokkaido Offshore Iburi 26 September 2012 Offshore Hokkaido 䝬䝎䝷 21 8.5 12 Marine Ecology Research Institute (Gadus macrocephalus) Pacific Ocean, Pacific Ocean, Offshore Chum salmon 9936 Hokkaido 1 October 2012 Hokkaido 21 September 2012 Offshore Hokkaido 䝅䝻䝄䜿 Not detectable Not detectable (<0.44) Not detectable (<0.35) Tohoku Ryokka Kankyohozen Hokkaido and Aomori (Oncorhynchus keta) and Aomori Chum salmon 9937 Hokkaido 1 October 2012 Hokkaido Offshore Oshima 21 September 2012 䠉 䝅䝻䝄䜿 Not detectable Not detectable (<0.52) Not detectable (<0.59) Mizuken, co.