Regnault, S., Dixon, J., Warren-Smith, C. M. R., Hutchinson, JR., & Weller
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J
Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J. Knüsel To cite this version: Sébastien Villotte, Christopher J. Knüsel. Understanding Entheseal Changes: Definition and Life Course Changes. International Journal of Osteoarchaeology, Wiley, 2013, Entheseal Changes and Occupation: Technical and Theoretical Advances and Their Applications, 23 (2), pp.135-146. 10.1002/oa.2289. hal-03147090 HAL Id: hal-03147090 https://hal.archives-ouvertes.fr/hal-03147090 Submitted on 19 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. International Journal of Osteoarchaeology Understanding Entheseal Changes: Definition and Life Course Changes Journal: International Journal of Osteoarchaeology Manuscript ID: OA-12-0089.R1 Wiley - ManuscriptFor type: Commentary Peer Review Date Submitted by the Author: n/a Complete List of Authors: Villotte, Sébastien; University of Bradford, AGES Knusel, Chris; University of Exeter, Department of Archaeology entheses, enthesopathy, Musculoskeletal Stress Markers (MSM), Keywords: senescence, activity, hormones, animal models, clinical studies http://mc.manuscriptcentral.com/oa Page 1 of 27 International Journal of Osteoarchaeology 1 2 3 Title: 4 5 Understanding Entheseal Changes: Definition and Life Course Changes 6 7 8 Short title: 9 10 Understanding Entheseal Changes 11 12 13 Keywords: entheses; enthesopathy; Musculoskeletal Stress Markers (MSM); senescence; 14 15 activity; hormones; animal models; clinical studies 16 17 18 Authors: For Peer Review 19 20 Villotte S. -

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

9 Impingement and Rotator Cuff Disease
Impingement and Rotator Cuff Disease 121 9 Impingement and Rotator Cuff Disease A. Stäbler CONTENTS Shoulder pain and chronic reduced function are fre- quently heard complaints in an orthopaedic outpa- 9.1 Defi nition of Impingement Syndrome 122 tient department. The symptoms are often related to 9.2 Stages of Impingement 123 the unique anatomic relationships present around the 9.3 Imaging of Impingement Syndrome: Uri Imaging Modalities 123 glenohumeral joint ( 1997). Impingement of the 9.3.1 Radiography 123 rotator cuff and adjacent bursa between the humeral 9.3.2 Ultrasound 126 head and the coracoacromial arch are among the most 9.3.3 Arthrography 126 common causes of shoulder pain. Neer noted that 9.3.4 Magnetic Resonance Imaging 127 elevation of the arm, particularly in internal rotation, 9.3.4.1 Sequences 127 9.3.4.2 Gadolinium 128 causes the critical area of the cuff to pass under the 9.3.4.3 MR Arthrography 128 coracoacromial arch. In cadaver dissections he found 9.4 Imaging Findings in Impingement Syndrome alterations attributable to mechanical impingement and Rotator Cuff Tears 130 including a ridge of proliferative spurs and excres- 9.4.1 Bursal Effusion 130 cences on the undersurface of the anterior margin 9.4.2 Imaging Following Impingement Test Injection 131 Neer Neer 9.4.3 Tendinosis 131 of the acromion ( 1972). Thus it was who 9.4.4 Partial Thickness Tears 133 introduced the concept of an impingement syndrome 9.4.5 Full-Thickness Tears 134 continuum ranging from chronic bursitis and partial 9.4.5.1 Subacromial Distance 136 tears to complete tears of the supraspinatus tendon, 9.4.5.2 Peribursal Fat Plane 137 which may extend to involve other parts of the cuff 9.4.5.3 Intramuscular Cysts 137 Neer Matsen 9.4.6 Massive Tears 137 ( 1972; 1990). -
Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079)
Local Coverage Article: Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, A and B MAC 01111 - MAC A J - E California - Entire State LLC Noridian Healthcare Solutions, A and B MAC 01112 - MAC B J - E California - Northern LLC Noridian Healthcare Solutions, A and B MAC 01182 - MAC B J - E California - Southern LLC Noridian Healthcare Solutions, A and B MAC 01211 - MAC A J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01212 - MAC B J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01311 - MAC A J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01312 - MAC B J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01911 - MAC A J - E American Samoa LLC California - Entire State Guam Hawaii Nevada Northern Mariana Created on 09/28/2019. Page 1 of 33 CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Islands Article Information General Information Original Effective Date 10/01/2019 Article ID Revision Effective Date A57079 N/A Article Title Revision Ending Date Billing and Coding: Injections - Tendon, Ligament, N/A Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma Retirement Date N/A Article Type Billing and Coding AMA CPT / ADA CDT / AHA NUBC Copyright Statement CPT codes, descriptions and other data only are copyright 2018 American Medical Association. -

Enthesitis of the Hands in Psoriatic Arthritis: an Ultrasonographic Perspective
Pictorial essay Med Ultrason 2017, Vol. 19, no. 4, 438-443 DOI: 10.11152/mu-1172 Enthesitis of the hands in psoriatic arthritis: an ultrasonographic perspective Alen Zabotti1, Luca Idolazzi2, Alberto Batticciotto3, Orazio De Lucia4, Carlo Alberto Scirè5, Ilaria Tinazzi6, Annamaria Iagnocco7 1Rheumatology Clinic, Department of Medical and Biological Sciences, University Hospital Santa Maria della Misericordia, Udine, 2Rheumatology Unit, University of Verona, Ospedale Civile Maggiore, Verona, 3Rheumatology Unit, L. Sacco University Hospital, Milan, 4Department of Rheumatology, ASST Centro traumatologico ortopedico G. Pini – CTO, Milan, 5Department of Medical Sciences, Section of Rheumatology, University of Ferrara, Ferrara, 6Unit of Rheumatology, Ospedale Sacro Cuore, Negrar, Verona, 7Dipartimento di Scienze Cliniche e Biologiche, Università degli Studi di Torino, Turin, Italy Abstract Psoriatic arthritis is a systemic inflammatory disease in which enthesitis and dactylitis are two of the main hallmarks of the disease. In the last years, ultrasonography is increasingly playing a key role in the diagnosis of psoriatic arthritis and ultrasonography of the entheses, particularly of the lower limbs, is commonly used to assess patients with that disease. New advancements in ultrasound equipment using high frequencies probes allowed us also to identify and characterize the involve- ment of the entheses of the hand in psoriatic arthritis, confirming the results of the experimental models of the disease and the theory of the sinovial-entheseal complex, even in small joints. Keywords: ultrasonography; psoriatic arthritis; enthesitis; seronegative arthritis; synovio-entheseal complex Introduction fulness to differentiate PsA from Rheumatoid Arthritis (RA) [4,5]. The European League Against Rheumatism Psoriatic Arthritis (PsA), usually included in the (EULAR) recommends the use of imaging in diagnosis Spondyloarthritis (SpA) group, can affect different ar- and management of SpA and, in the last years, ultrasound ticular structures, from bone to soft tissues (e.g. -

Enthesopathy and Tendinopathy in Gout: Computed Tomographic Assessment
Ann Rheum Dis 1996;55:921-923 921 CONCISE REPORTS Ann Rheum Dis: first published as 10.1136/ard.55.12.921 on 1 December 1996. Downloaded from Enthesopathy and tendinopathy in gout: computed tomographic assessment Jean-Charles Gerster, Michel Landry, Georges Rappoport, Gilles Rivier, Bertrand Duvoisin, Pierre Schnyder Abstract urate deposits in clinically involved tendons Objective-To establish if computed (Achilles tendon in two patients, patellar tendon tomography (CT) imaging, which has in one patient) was assessed. proved helpful in detecting intra-articular tophi in gout, can also be used to Case reports document gouty enthesopathy and tendin- PATIENT 1 opathy. A 70 year old man was admitted with acute Methods-Three patients with tophaceous arthritis of the left ankle joint. He had been gout and clinical involvement of the suffering from gout for 10 years, and had a his- Achilles tendon (two cases) or patellar tory of excessive alcohol consumption and of tendon (one case) were assessed with CT irregular medication consisting of non- examination and plain radiographs. steroidal anti-inflammatory drugs and allopu- Results-In the first two cases, CT images rinol. revealed linear or nodular high attenua- Upon admission, the patient was overweight tion opacities within the substance of the (body mass index 32.8 kg m-', normal < 25), Achilles tendons and their calcaneal and he had an effusion of the left knee, signs of insertion. In case 3, dense linear opacities acute arthritis of the left ankle, and nodules of were seen within the patellar tendon and both Achilles tendons, which were slightly ten- within its tibial insertion. -

The Correlations Between Dimensions of the Normal Tendon And
www.nature.com/scientificreports OPEN The correlations between dimensions of the normal tendon and tendinopathy changed Achilles tendon in routine magnetic resonance imaging Pawel Szaro 1,2,3* & Khaldun Ghali Gataa2 This comparative study aimed to investigate how tendinopathy-related lesions change correlations in the dimensions of the Achilles tendon. Our experimental group included 74 patients. The mean age was 52.9 ± 10.4 years. The control group included 81 patients with a mean age was 35.2 ± 13.6 years, p < .001. The most signifcant diference in correlation was the thickness of the tendon and the midportion’s width, which was more signifcant in the tendinopathy (r = .49 vs. r = .01, p < .001). The correlation was positive between width and length of the insertion but negative in normal tendons (r = .21 vs. r = − .23, p < .001). The correlation was between the midportions width in tendinopathy and the tendon’s length but negative in the normal tendon (r = .16 vs. r = − .23, p < .001). The average thickness of the midportion in tendinopathy was 11.2 ± 3.3 mm, and 4.9 ± 0.5 mm in the control group, p < .001. The average width of the midportion and insertion was more extensive in the experimental group, 17.2 ± 3.1 mm vs. 14.7 ± 1.8 mm for the midportion and 31.0 ± 3.9 mm vs. 25.7 ± 3.0 mm for insertion, respectively, p < .001. The tendon’s average length was longer in tendinopathy (83.5 ± 19.3 mm vs. 61.5 ± 14.4 mm, p < .001). The dimensions correlations in normal Achilles tendon and tendinopathic tendon difer signifcantly. -
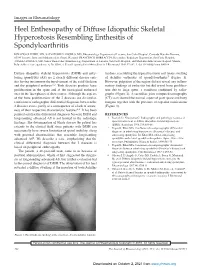
Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis
Images in Rheumatology Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis Resembling Enthesitis of Spondyloarthritis IGNAZIO OLIVIERI, MD, SALVATORE D’ANGELO, MD, Rheumatology Department of Lucania, San Carlo Hospital, Contrada Macchia Romana, 85100 Potenza, Italy; and Madonna delle Grazie Hospital; FRANCESCO BORRACCIA, Researcher, Radiology Department, San Carlo Hospital; ANGELA PADULA, MD, Senior Researcher, Rheumatology Department of Lucania, San Carlo Hospital, and Madonna delle Grazie Hospital, Matera, Italy. Address correspondence to Dr. Olivieri; E-mail: [email protected]. J Rheumatol 2010;37:192–3; doi.10.3899/jrheum.090514 Diffuse idiopathic skeletal hyperostosis (DISH) and anky- tendons, resembling the typical fusiform soft tissue swelling losing spondylitis (AS) are 2 clearly different disease enti- of Achilles enthesitis of spondyloarthritis5 (Figure 1). ties having in common the involvement of the axial skeleton However, palpation of the region did not reveal any inflam- and the peripheral entheses1,2. Both diseases produce bone matory findings of enthesitis but did reveal bone prolifera- proliferation in the spine and at the extraspinal entheseal tion due to large spurs, a condition confirmed by radio- sites in the later phases of their course. Although the aspects graphs (Figure 2). A sacroiliac joint computed tomography of the bone proliferations of the 2 diseases are dissimilar, (CT) scan showed the normal aspect of joint space and bony confusion of radiographic differential diagnosis between the margins together with the presence of capsular ossifications 2 diseases exists, partly as a consequence of a lack of aware- (Figure 3). ness of their respective characteristic features2,3. It has been pointed out that the differential diagnosis between DISH and REFERENCES longstanding advanced AS is not limited to the radiologic 1. -

Tendinopathy: Tackling Troubled Tendons
Tendinopathy: Tackling Troubled Tendons Deepak Patel, MD, FAAFP, FACSM ACTIVITY DISCLAIMER The material presented here is being made available by the American Academy of Family Physicians for educational purposes only. Please note that medical information is constantly changing; the information contained in this activity was accurate at the time of publication. This material is not intended to represent the only, nor necessarily best, methods or procedures appropriate for the medical situations discussed. Rather, it is intended to present an approach, view, statement, or opinion of the faculty, which may be helpful to others who face similar situations. The AAFP disclaims any and all liability for injury or other damages resulting to any individual using this material and for all claims that might arise out of the use of the techniques demonstrated therein by such individuals, whether these claims shall be asserted by a physician or any other person. Physicians may care to check specific details such as drug doses and contraindications, etc., in standard sources prior to clinical application. This material might contain recommendations/guidelines developed by other organizations. Please note that although these guidelines might be included, this does not necessarily imply the endorsement by the AAFP. 1 DISCLOSURE It is the policy of the AAFP that all individuals in a position to control content disclose any relationships with commercial interests upon nomination/invitation of participation. Disclosure documents are reviewed for potential conflict of interest (COI), and if identified, conflicts are resolved prior to confirmation of participation. Only those participants who had no conflict of interest or who agreed to an identified resolution process prior to their participation were involved in this CME activity. -

Elbow Tendonopathy & Enthesopathy Outline
7/7/2017 Elbow Tendonopathy & Outline Enthesopathy • Medial epicondylitis • Medial elbow “snapping” Patrick H. Smock, MD • Triceps enthesopathy Sports and Orthopedic Specialists 7 July, 2017 • Distal biceps tendinitis • Lateral epicondylitis Tendinopathy vs. Enthesopathy Medial Epicondylitis • “Tendinopathy refers to disease of the tendon and enthesopathy refer “Golfers elbow” to disease of the tendon bone junction (where the tendon joins the • 3‐6x less common than lat. Epicondylitis bone).... In the context of sports medicine, most of these conditions • Repetitive microtrauma (valgus stress are overuse in nature with repetitive loading leading to accumulative and wrist/forearm flexion/pronation) damage to the tendon or its insertion.” • “Angiofibroblastic hyperplasia” • Clinical features: Pain/tenderness over “With age, the circulation to some tendons decrease and this impairs medial epicondyle • Pain with grip and with resisted wrist the attempts of the body to repair the degenerated areas. flexion/pronation Tendinopathy and enthesopathy can also result in calcification within the tendon. In some cases, the progressive degeneration of the tendon • R/O Ulnar nerve pathology and medial or enthesis eventually causes the structure to fail and results in a tear.” elbow instability • Imaging as needed if diagnosis unclear Tendinopathies and enthesopathies: the bugbear of athletes • US vs. MRI https://www.ttsh.com.sg/patient‐guide/medical‐departments/page.aspx?id=1177 ©AllinaHealthSystem 1 7/7/2017 Management Medial elbow snapping • Conservative management is the mainstay • Rest, avoidance of offending activity, • Ulnar nerve vs. Medial triceps stretch, ice, NSAIDs tendon (or both) • Wrist splinting vs. counterforce strap • Painful snapping palpated/visualized with elbow • Shock‐wave therapy? flex/ext • Injections (CSI, autologous blood products)* • Check for subluxation of the Ulnar • Medial antebrachial cutaneous nerve nerve • Surgical debridement/repair (address associated ulnar nerve compression) • Synovial pathology Shoulder Elbow. -

A Narrative Review of the Classification and Use Of
diagnostics Review A Narrative Review of the Classification and Use of Diagnostic Ultrasound for Conditions of the Achilles Tendon Sheryl Mascarenhas Department of Internal Medicine, Division of Rheumatology, The Ohio State University Wexner Medical Center, 543 Taylor Ave, Columbus, OH 43203, USA; [email protected] Received: 15 September 2020; Accepted: 4 November 2020; Published: 13 November 2020 Abstract: Enthesitis is a cardinal feature of spondyloarthropathies. The Achilles insertion on the calcaneus is a commonly evaluated enthesis located at the hindfoot, generally resulting in hindfoot pain and possible tendon enlargement. For decades, diagnosis of enthesitis was based upon patient history of hindfoot or posterior ankle pain and clinical examination revealing tenderness and/or enlargement at the site of the tendon insertion. However, not all hindfoot or posterior ankle symptoms are related to enthesitis. Advanced imaging, including magnetic resonance imaging (MRI) and ultrasound (US), has allowed for more precise evaluation of hindfoot and posterior ankle conditions. Use of US in diagnosis has helped confirm some of these cases but also identified other conditions that may have otherwise been misclassified without use of advanced imaging diagnostics. Conditions that may result in hindfoot and posterior ankle symptoms related to the Achilles tendon include enthesitis (which can include retrocalcaneal bursitis and insertional tendonopathy), midportion tendonopathy, paratenonopathy, superficial calcaneal bursitis, calcaneal ossification (Haglund deformity), and calcific tendonopathy. With regard to classification of these conditions, much of the existing literature uses confusing nomenclature to describe conditions in this region of the body. Some terminology may imply inflammation when in fact there may be none. A more uniform approach to classifying these conditions based off anatomic location, symptoms, clinical findings, and histopathology is needed. -

Musculoskeletal Embolization Inflammatory and Degenerative Disease
Musculoskeletal embolization Inflammatory and degenerative Disease Yuji Okuno Musculoskeletal Intervention Center Edogawa Hospital Conflict of interest: none Tokyo, JAPAN J Vasc Interv Radiol 2013 June ; 24: 787-792 J Vasc Interv Radiol 2013 June ; 24: 787-792 • Tendinopathy and enthesopathy Lateral Epicondylitis (“Tennis Elbow”) Patellar Tendinopathy (“Jumpers’ Knee”) Achilles Tendinopathy etc Case: Patellar tendinopathy 58y.o. male High level long distance city runner 350km / month before disease onset Due to his pain, he could not run for 10 months Case: Patellar tendinopathy Patellar tendon Patellar tendon Affected side Unaffected side Selective angiography of lateral inferior genicular artery Before TAE Patellar Normal appearance of lateral inferior genicular artery Normal Knee patellar Selective angiography of lateral inferior genicular artery Before Embolization Patellar Selective angiography of lateral inferior genicular artery After Embolization Patellar Change of Pain Score Loxoprofen 180mg/day Physical Therapy Embolization 100 80 60 VAS (mm) VAS 40 20 Pain 0 2012 2013 2014 2016 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 1 2 7 (month) 1year follow up of first 12 patients with tendinopathy and enthesopathy Our MSK Embolization from 2012 to 2016 June • Tendinopathy and enthesopathy 98 cases • MSK shoulder pain (frozen shoulder etc) 128 cases • Knee osteoarthritis 95 cases • Sports injuries 44 cases • Persistent pain after joint replacement 32 cases • Others (hip, ankle, wrist, elbow, etc) 152cases total n = 549 Today’s