The Correlations Between Dimensions of the Normal Tendon And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J
Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J. Knüsel To cite this version: Sébastien Villotte, Christopher J. Knüsel. Understanding Entheseal Changes: Definition and Life Course Changes. International Journal of Osteoarchaeology, Wiley, 2013, Entheseal Changes and Occupation: Technical and Theoretical Advances and Their Applications, 23 (2), pp.135-146. 10.1002/oa.2289. hal-03147090 HAL Id: hal-03147090 https://hal.archives-ouvertes.fr/hal-03147090 Submitted on 19 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. International Journal of Osteoarchaeology Understanding Entheseal Changes: Definition and Life Course Changes Journal: International Journal of Osteoarchaeology Manuscript ID: OA-12-0089.R1 Wiley - ManuscriptFor type: Commentary Peer Review Date Submitted by the Author: n/a Complete List of Authors: Villotte, Sébastien; University of Bradford, AGES Knusel, Chris; University of Exeter, Department of Archaeology entheses, enthesopathy, Musculoskeletal Stress Markers (MSM), Keywords: senescence, activity, hormones, animal models, clinical studies http://mc.manuscriptcentral.com/oa Page 1 of 27 International Journal of Osteoarchaeology 1 2 3 Title: 4 5 Understanding Entheseal Changes: Definition and Life Course Changes 6 7 8 Short title: 9 10 Understanding Entheseal Changes 11 12 13 Keywords: entheses; enthesopathy; Musculoskeletal Stress Markers (MSM); senescence; 14 15 activity; hormones; animal models; clinical studies 16 17 18 Authors: For Peer Review 19 20 Villotte S. -

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

Atraumatic Bilateral Achilles Tendon Rupture: an Association of Systemic
378 Kotnis, Halstead, Hormbrey Acute compartment syndrome may be a of the body of gastrocnemius has been result of any trauma to the limb. The trauma is reported in athletes.7 8 This, however, is the J Accid Emerg Med: first published as 10.1136/emj.16.5.378 on 1 September 1999. Downloaded from usually a result of an open or closed fracture of first reported case of acute compartment the bones, or a crush injury to the limb. Other syndrome caused by a gastrocnemius muscle causes include haematoma, gun shot or stab rupture in a non-athlete. wounds, animal or insect bites, post-ischaemic swelling, vascular damage, electrical injuries, burns, prolonged tourniquet times, etc. Other Conclusion causes of compartment syndrome are genetic, Soft tissue injuries and muscle tears occur fre- iatrogenic, or acquired coagulopathies, infec- quently in athletes. Most injuries result from tion, nephrotic syndrome or any cause of direct trauma. Indirect trauma resulting in decreased tissue osmolarity and capillary per- muscle tears and ruptures can cause acute meability. compartment syndrome in athletes. It is also Chronic compartment syndrome is most important to keep in mind the possibility of typically an exercise induced condition charac- similar injuries in a non-athlete as well. More terised by a relative inadequacy of musculofas- research is needed to define optimal manage- cial compartment size producing chronic or ment patterns and potential strategies for recurring pain and/or disability. It is seen in injury prevention. athletes, who often have recurring leg pain that Conflict of interest: none. starts after they have been exercising for some Funding: none. -

9 Impingement and Rotator Cuff Disease
Impingement and Rotator Cuff Disease 121 9 Impingement and Rotator Cuff Disease A. Stäbler CONTENTS Shoulder pain and chronic reduced function are fre- quently heard complaints in an orthopaedic outpa- 9.1 Defi nition of Impingement Syndrome 122 tient department. The symptoms are often related to 9.2 Stages of Impingement 123 the unique anatomic relationships present around the 9.3 Imaging of Impingement Syndrome: Uri Imaging Modalities 123 glenohumeral joint ( 1997). Impingement of the 9.3.1 Radiography 123 rotator cuff and adjacent bursa between the humeral 9.3.2 Ultrasound 126 head and the coracoacromial arch are among the most 9.3.3 Arthrography 126 common causes of shoulder pain. Neer noted that 9.3.4 Magnetic Resonance Imaging 127 elevation of the arm, particularly in internal rotation, 9.3.4.1 Sequences 127 9.3.4.2 Gadolinium 128 causes the critical area of the cuff to pass under the 9.3.4.3 MR Arthrography 128 coracoacromial arch. In cadaver dissections he found 9.4 Imaging Findings in Impingement Syndrome alterations attributable to mechanical impingement and Rotator Cuff Tears 130 including a ridge of proliferative spurs and excres- 9.4.1 Bursal Effusion 130 cences on the undersurface of the anterior margin 9.4.2 Imaging Following Impingement Test Injection 131 Neer Neer 9.4.3 Tendinosis 131 of the acromion ( 1972). Thus it was who 9.4.4 Partial Thickness Tears 133 introduced the concept of an impingement syndrome 9.4.5 Full-Thickness Tears 134 continuum ranging from chronic bursitis and partial 9.4.5.1 Subacromial Distance 136 tears to complete tears of the supraspinatus tendon, 9.4.5.2 Peribursal Fat Plane 137 which may extend to involve other parts of the cuff 9.4.5.3 Intramuscular Cysts 137 Neer Matsen 9.4.6 Massive Tears 137 ( 1972; 1990). -
Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079)
Local Coverage Article: Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, A and B MAC 01111 - MAC A J - E California - Entire State LLC Noridian Healthcare Solutions, A and B MAC 01112 - MAC B J - E California - Northern LLC Noridian Healthcare Solutions, A and B MAC 01182 - MAC B J - E California - Southern LLC Noridian Healthcare Solutions, A and B MAC 01211 - MAC A J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01212 - MAC B J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01311 - MAC A J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01312 - MAC B J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01911 - MAC A J - E American Samoa LLC California - Entire State Guam Hawaii Nevada Northern Mariana Created on 09/28/2019. Page 1 of 33 CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Islands Article Information General Information Original Effective Date 10/01/2019 Article ID Revision Effective Date A57079 N/A Article Title Revision Ending Date Billing and Coding: Injections - Tendon, Ligament, N/A Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma Retirement Date N/A Article Type Billing and Coding AMA CPT / ADA CDT / AHA NUBC Copyright Statement CPT codes, descriptions and other data only are copyright 2018 American Medical Association. -

Juvenile Spondyloarthritis / Enthesitis Related Arthritis (Spa-ERA) Version of 2016
https://www.printo.it/pediatric-rheumatology/GB/intro Juvenile Spondyloarthritis / Enthesitis Related Arthritis (SpA-ERA) Version of 2016 1. WHAT IS JUVENILE SPONDYLOARTHRITIS/ENTHESITIS- RELATED ARTHRITIS (SpA-ERA) 1.1 What is it? Juvenile SpA-ERA constitutes a group of chronic inflammatory diseases of the joints (arthritis), as well as tendon and ligament attachments to certain bones (enthesitis) and affects predominantly the lower limbs and in some cases the pelvic and spinal joints (sacroiliitis - buttock pain and spondylitis - back pain). Juvenile SpA-ERA is significantly more common in people that have a positive blood test for the genetic factor HLA-B27. HLA-B27 is a protein located on the surface of immune cells. Remarkably, only a fraction of people with HLA-B27 ever develops arthritis. Thus, the presence of HLA-B27 is not enough to explain the development of the disease. To date, the exact role of HLA-B27 in the origin of the disease remains unknown. However, it is known that in very few cases the onset of arthritis is preceded by gastrointestinal or urogenital infection (known as reactive arthritis). Juvenile SpA-ERA is closely related to the spondyloarthritis with onset in adulthood and most researchers believe these diseases share the same origin and characteristics. Most children and adolescents with juvenile spondyloarthritis would be diagnosed as affected by ERA and even psoriatic arthritis. It is important that the names "juvenile spondyloarthritis", "enthesitis-related arthritis" and in some cases "psoriatic arthritis" may be the same from a clinical and therapeutic point of view. 1 / 12 1.2 What diseases are called juvenile SpA-ERA? As mentioned above, juvenile spondyloarthritis is the name for a group of diseases; the clinical features may overlap with each other, including axial and peripheral spondyloarthritis, ankylosing spondylitis, undifferentiated spondyloarthritis, psoriatic arthritis, reactive arthritis and arthritis associated with Crohn’s disease and ulcerative colitis. -

Enthesitis of the Hands in Psoriatic Arthritis: an Ultrasonographic Perspective
Pictorial essay Med Ultrason 2017, Vol. 19, no. 4, 438-443 DOI: 10.11152/mu-1172 Enthesitis of the hands in psoriatic arthritis: an ultrasonographic perspective Alen Zabotti1, Luca Idolazzi2, Alberto Batticciotto3, Orazio De Lucia4, Carlo Alberto Scirè5, Ilaria Tinazzi6, Annamaria Iagnocco7 1Rheumatology Clinic, Department of Medical and Biological Sciences, University Hospital Santa Maria della Misericordia, Udine, 2Rheumatology Unit, University of Verona, Ospedale Civile Maggiore, Verona, 3Rheumatology Unit, L. Sacco University Hospital, Milan, 4Department of Rheumatology, ASST Centro traumatologico ortopedico G. Pini – CTO, Milan, 5Department of Medical Sciences, Section of Rheumatology, University of Ferrara, Ferrara, 6Unit of Rheumatology, Ospedale Sacro Cuore, Negrar, Verona, 7Dipartimento di Scienze Cliniche e Biologiche, Università degli Studi di Torino, Turin, Italy Abstract Psoriatic arthritis is a systemic inflammatory disease in which enthesitis and dactylitis are two of the main hallmarks of the disease. In the last years, ultrasonography is increasingly playing a key role in the diagnosis of psoriatic arthritis and ultrasonography of the entheses, particularly of the lower limbs, is commonly used to assess patients with that disease. New advancements in ultrasound equipment using high frequencies probes allowed us also to identify and characterize the involve- ment of the entheses of the hand in psoriatic arthritis, confirming the results of the experimental models of the disease and the theory of the sinovial-entheseal complex, even in small joints. Keywords: ultrasonography; psoriatic arthritis; enthesitis; seronegative arthritis; synovio-entheseal complex Introduction fulness to differentiate PsA from Rheumatoid Arthritis (RA) [4,5]. The European League Against Rheumatism Psoriatic Arthritis (PsA), usually included in the (EULAR) recommends the use of imaging in diagnosis Spondyloarthritis (SpA) group, can affect different ar- and management of SpA and, in the last years, ultrasound ticular structures, from bone to soft tissues (e.g. -

Enthesopathy and Tendinopathy in Gout: Computed Tomographic Assessment
Ann Rheum Dis 1996;55:921-923 921 CONCISE REPORTS Ann Rheum Dis: first published as 10.1136/ard.55.12.921 on 1 December 1996. Downloaded from Enthesopathy and tendinopathy in gout: computed tomographic assessment Jean-Charles Gerster, Michel Landry, Georges Rappoport, Gilles Rivier, Bertrand Duvoisin, Pierre Schnyder Abstract urate deposits in clinically involved tendons Objective-To establish if computed (Achilles tendon in two patients, patellar tendon tomography (CT) imaging, which has in one patient) was assessed. proved helpful in detecting intra-articular tophi in gout, can also be used to Case reports document gouty enthesopathy and tendin- PATIENT 1 opathy. A 70 year old man was admitted with acute Methods-Three patients with tophaceous arthritis of the left ankle joint. He had been gout and clinical involvement of the suffering from gout for 10 years, and had a his- Achilles tendon (two cases) or patellar tory of excessive alcohol consumption and of tendon (one case) were assessed with CT irregular medication consisting of non- examination and plain radiographs. steroidal anti-inflammatory drugs and allopu- Results-In the first two cases, CT images rinol. revealed linear or nodular high attenua- Upon admission, the patient was overweight tion opacities within the substance of the (body mass index 32.8 kg m-', normal < 25), Achilles tendons and their calcaneal and he had an effusion of the left knee, signs of insertion. In case 3, dense linear opacities acute arthritis of the left ankle, and nodules of were seen within the patellar tendon and both Achilles tendons, which were slightly ten- within its tibial insertion. -
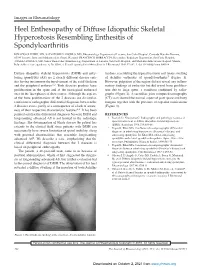
Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis
Images in Rheumatology Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis Resembling Enthesitis of Spondyloarthritis IGNAZIO OLIVIERI, MD, SALVATORE D’ANGELO, MD, Rheumatology Department of Lucania, San Carlo Hospital, Contrada Macchia Romana, 85100 Potenza, Italy; and Madonna delle Grazie Hospital; FRANCESCO BORRACCIA, Researcher, Radiology Department, San Carlo Hospital; ANGELA PADULA, MD, Senior Researcher, Rheumatology Department of Lucania, San Carlo Hospital, and Madonna delle Grazie Hospital, Matera, Italy. Address correspondence to Dr. Olivieri; E-mail: [email protected]. J Rheumatol 2010;37:192–3; doi.10.3899/jrheum.090514 Diffuse idiopathic skeletal hyperostosis (DISH) and anky- tendons, resembling the typical fusiform soft tissue swelling losing spondylitis (AS) are 2 clearly different disease enti- of Achilles enthesitis of spondyloarthritis5 (Figure 1). ties having in common the involvement of the axial skeleton However, palpation of the region did not reveal any inflam- and the peripheral entheses1,2. Both diseases produce bone matory findings of enthesitis but did reveal bone prolifera- proliferation in the spine and at the extraspinal entheseal tion due to large spurs, a condition confirmed by radio- sites in the later phases of their course. Although the aspects graphs (Figure 2). A sacroiliac joint computed tomography of the bone proliferations of the 2 diseases are dissimilar, (CT) scan showed the normal aspect of joint space and bony confusion of radiographic differential diagnosis between the margins together with the presence of capsular ossifications 2 diseases exists, partly as a consequence of a lack of aware- (Figure 3). ness of their respective characteristic features2,3. It has been pointed out that the differential diagnosis between DISH and REFERENCES longstanding advanced AS is not limited to the radiologic 1. -

Clinical and Imaging Assessment of Peripheral Enthesitis in Ankylosing Spondylitis
Special RepoRt Clinical and imaging assessment of peripheral enthesitis in ankylosing spondylitis Enthesitis, defined as inflammation of the origin and insertion of ligaments, tendons, aponeuroses, annulus fibrosis and joint capsules, is a hallmark of ankylosing spondylitis. The concept of entheseal organ prone to pathological changes in ankylosing spondylitis and other spondyloarthritis is well recognized. The relevant role of peripheral enthesitis is supported by the evidence that this feature, on clinical examination, has been included in the classification criteria of Amor (heel pain or other well-defined enthesopathic pain), European Spondiloarthropathy Study Group and Assessment in SpondyloArthritis International Society for axial and peripheral spondyloarthritis. Nevertheless, the assessment of enthesitis has been improved by imaging techniques to carefully detect morphological abnormalities and to monitor disease activity. 1 Keywords: ankylosing spondylitis n clinical assessment n enthesitis n MrI Antonio Spadaro* , n spondyloarthritis n ultrasound Fabio Massimo Perrotta1, In primary ankylosing spondylitis (AS) the fre- has proven to be a highly sensitive and nonin- Alessia Carboni1 quency of peripheral enthesitis has been found vasive tool to assess the presence of enthesitis, & Antongiulio Scarno1 to be between 25 and 58% [1], however, the real characterized by hypoechogenicity with loss 1Dipartimento di Medicina Interna e prevalence of this feature depends on the type of tendon fibrillar pattern, tendon thickening, Specialità -

Foot Pain & Psoriatic Arthritis
WHEATON • ROCKVILLE • CHEVY CHASE • WASHINGTON, DC Foot Pain & Psoriatic Arthritis Daniel El-Bogdadi, MD, FACR Arthritis and Rheumatism Associates, P.C. Do you feel heel pain in the Plantar fasciitis ARTHRITIS morning or after a period of can be caused by inactivity? This might be an a number AND indication of plantar fasciitis that of factors, RHEUMATISM could be part of an underlying including aerobic ASSOCIATES, P.C. psoriatic arthritis condition. dance exercise, running, and Board Certified Rheumatologists Psoriatic arthritis is an inflammatory ballet. arthritis that typically causes pain, Herbert S.B. Baraf MD FACP MACR swelling and stiffness in the Robert L. Rosenberg peripheral joints or spine. However, MD FACR CCD if you have psoriatic arthritis, you Evan L. Siegel may notice that not only are the MD FACR joints and spine involved, but Emma DiIorio symptoms may also occur in the soft MD FACR tissue such as tendons or ligaments. David G. Borenstein MD MACP MACR This usually happens in a place Alan K. Matsumoto where tendons and ligaments attach There are several treatment options MD FACP FACR to bone, known as the enthesis. for plantar fasciitis: David P. Wolfe When this attachment gets inflamed MD FACR it is called enthesitis. • The first immediate intervention is Paul J. DeMarco MD FACP FACR to decrease or stop any Shari B. Diamond One of the more common areas to repetitive activities, such as MD FACP FACR get enthesitis is in the Achilles running or dancing, which may Ashley D. Beall tendon. Another common area for aggravate the condition. MD FACR inflammation is in thick band of (over) Angus B. -

Spondylitis Diseases Psoriasis
CD8 T-cell Spondylitis leads to the development of syndesmophytes and ankylosis Spondyloarthritis Diseases Cognitive ASp Recognition TCR •A group of autoimmune Unknown Self Antigenic Peptide diseases that in common Presented by class I MHC appear mediated by activation Target Cell of autoreactive CD8 T cells •Physical stress, inflammation and infection with specific microorganisms trigger the immune response •Primarily affect joints, skin, eyes and mucous membranes T cells invade the junction Annulus fibers are eroded, Progressive of annulus fibrosis and then replaced by fibrocartilage cartilaginous and vertebral body forming that ossifies to form a periosteal ossification •Autoantibodies such as ANA or RF are absent granulation tissue syndesmophyte. Subperiosteal forms a “bamboo spine”, (activated macrophages, T new bone formation ensues osteoporosis develops cells and fibroblasts) Spondylitis Diseases Sacroiliitis DAnkylosing spondylitis (ASp) DReiter’s syndrome (RS) / reactive arthritis (ReA) DPsoriatic arthritis (PsA) Undifferentiated spondyloarthritis (USpA) Enteropathic arthritis (ulcerative colitis, regional enteritis) The subchondral regions of the The cartilage on the iliac side is eroded synarthrotic SI joints are first, causing bone plate blurring, joint invaded by T cells leading to space “widening” and reactive sclerosis. Psoriasis the formulation of granulation Ultimately the resultant fibrous ankylosis tissue is replaced by bone, obliterating the SI joint Spondyloarthritis Diseases-features common to all Enthesitis (enthesopathy) the central inflammatory unit of spondyloarthritis Entheses are the specialized fibrocartilagenous region of bone 1. Clinical:- Affect joints, skin, eyes and mucous membranes in where ligaments, tendons, fascia or joint capsules insert varying proportions with characteristic joint involvement: Spondylitis (inflammation of vertebral discs), sacroiliitis (sacroiliac joints) and enthesitis (tendon insertions).