A Narrative Review of the Classification and Use Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J
Understanding Entheseal Changes: Definition and Life Course Changes Sébastien Villotte, Christopher J. Knüsel To cite this version: Sébastien Villotte, Christopher J. Knüsel. Understanding Entheseal Changes: Definition and Life Course Changes. International Journal of Osteoarchaeology, Wiley, 2013, Entheseal Changes and Occupation: Technical and Theoretical Advances and Their Applications, 23 (2), pp.135-146. 10.1002/oa.2289. hal-03147090 HAL Id: hal-03147090 https://hal.archives-ouvertes.fr/hal-03147090 Submitted on 19 Feb 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. International Journal of Osteoarchaeology Understanding Entheseal Changes: Definition and Life Course Changes Journal: International Journal of Osteoarchaeology Manuscript ID: OA-12-0089.R1 Wiley - ManuscriptFor type: Commentary Peer Review Date Submitted by the Author: n/a Complete List of Authors: Villotte, Sébastien; University of Bradford, AGES Knusel, Chris; University of Exeter, Department of Archaeology entheses, enthesopathy, Musculoskeletal Stress Markers (MSM), Keywords: senescence, activity, hormones, animal models, clinical studies http://mc.manuscriptcentral.com/oa Page 1 of 27 International Journal of Osteoarchaeology 1 2 3 Title: 4 5 Understanding Entheseal Changes: Definition and Life Course Changes 6 7 8 Short title: 9 10 Understanding Entheseal Changes 11 12 13 Keywords: entheses; enthesopathy; Musculoskeletal Stress Markers (MSM); senescence; 14 15 activity; hormones; animal models; clinical studies 16 17 18 Authors: For Peer Review 19 20 Villotte S. -

Iliopsoas Tendonitis/Bursitis Exercises
ILIOPSOAS TENDONITIS / BURSITIS What is the Iliopsoas and Bursa? The iliopsoas is a muscle that runs from your lower back through the pelvis to attach to a small bump (the lesser trochanter) on the top portion of the thighbone near your groin. This muscle has the important job of helping to bend the hip—it helps you to lift your leg when going up and down stairs or to start getting out of a car. A fluid-filled sac (bursa) helps to protect and allow the tendon to glide during these movements. The iliopsoas tendon can become inflamed or overworked during repetitive activities. The tendon can also become irritated after hip replacement surgery. Signs and Symptoms Iliopsoas issues may feel like “a pulled groin muscle”. The main symptom is usually a catch during certain movements such as when trying to put on socks or rising from a seated position. You may find yourself leading with your other leg when going up the stairs to avoid lifting the painful leg. The pain may extend from the groin to the inside of the thigh area. Snapping or clicking within the front of the hip can also be experienced. Do not worry this is not your hip trying to pop out of socket but it is usually the iliopsoas tendon rubbing over the hip joint or pelvis. Treatment Conservative treatment in the form of stretching and strengthening usually helps with the majority of patients with iliopsoas bursitis. This issue is the result of soft tissue inflammation, therefore rest, ice, anti- inflammatory medications, physical therapy exercises, and/or injections are effective treatment options. -

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

Bioarchaeological Implications of Calcaneal Spurs in the Medieval Nubian Population of Kulubnarti
Bioarchaeological Implications of Calcaneal Spurs in the Medieval Nubian Population of Kulubnarti Lindsay Marker Department of Anthropology Primary Thesis Advisor Matthew Sponheimer, Department of Anthropology Defense Committee Members Douglas Bamforth, Department of Anthropology Patricia Sullivan, Department of English University of Colorado at Boulder April 2016 1 Table of Contents List of Figures ............................................................................................................................. 4 Abstract …................................................................................................................................... 6 Chapter 1: Introduction …........................................................................................................... 8 Chapter 2: Anatomy …................................................................................................................ 11 2.1 Chapter Overview …................................................................................................. 11 2.2 Bone Composition …................................................................................................ 11 2.3 Plantar Foot Anatomy …........................................................................................... 12 2.4 Posterior Foot Anatomy …........................................................................................ 15 Chapter 3: Literature Review and Background of Calcaneal Enthesophytes ............................. 18 3.1 Chapter Overview …................................................................................................ -

Atraumatic Bilateral Achilles Tendon Rupture: an Association of Systemic
378 Kotnis, Halstead, Hormbrey Acute compartment syndrome may be a of the body of gastrocnemius has been result of any trauma to the limb. The trauma is reported in athletes.7 8 This, however, is the J Accid Emerg Med: first published as 10.1136/emj.16.5.378 on 1 September 1999. Downloaded from usually a result of an open or closed fracture of first reported case of acute compartment the bones, or a crush injury to the limb. Other syndrome caused by a gastrocnemius muscle causes include haematoma, gun shot or stab rupture in a non-athlete. wounds, animal or insect bites, post-ischaemic swelling, vascular damage, electrical injuries, burns, prolonged tourniquet times, etc. Other Conclusion causes of compartment syndrome are genetic, Soft tissue injuries and muscle tears occur fre- iatrogenic, or acquired coagulopathies, infec- quently in athletes. Most injuries result from tion, nephrotic syndrome or any cause of direct trauma. Indirect trauma resulting in decreased tissue osmolarity and capillary per- muscle tears and ruptures can cause acute meability. compartment syndrome in athletes. It is also Chronic compartment syndrome is most important to keep in mind the possibility of typically an exercise induced condition charac- similar injuries in a non-athlete as well. More terised by a relative inadequacy of musculofas- research is needed to define optimal manage- cial compartment size producing chronic or ment patterns and potential strategies for recurring pain and/or disability. It is seen in injury prevention. athletes, who often have recurring leg pain that Conflict of interest: none. starts after they have been exercising for some Funding: none. -

9 Impingement and Rotator Cuff Disease
Impingement and Rotator Cuff Disease 121 9 Impingement and Rotator Cuff Disease A. Stäbler CONTENTS Shoulder pain and chronic reduced function are fre- quently heard complaints in an orthopaedic outpa- 9.1 Defi nition of Impingement Syndrome 122 tient department. The symptoms are often related to 9.2 Stages of Impingement 123 the unique anatomic relationships present around the 9.3 Imaging of Impingement Syndrome: Uri Imaging Modalities 123 glenohumeral joint ( 1997). Impingement of the 9.3.1 Radiography 123 rotator cuff and adjacent bursa between the humeral 9.3.2 Ultrasound 126 head and the coracoacromial arch are among the most 9.3.3 Arthrography 126 common causes of shoulder pain. Neer noted that 9.3.4 Magnetic Resonance Imaging 127 elevation of the arm, particularly in internal rotation, 9.3.4.1 Sequences 127 9.3.4.2 Gadolinium 128 causes the critical area of the cuff to pass under the 9.3.4.3 MR Arthrography 128 coracoacromial arch. In cadaver dissections he found 9.4 Imaging Findings in Impingement Syndrome alterations attributable to mechanical impingement and Rotator Cuff Tears 130 including a ridge of proliferative spurs and excres- 9.4.1 Bursal Effusion 130 cences on the undersurface of the anterior margin 9.4.2 Imaging Following Impingement Test Injection 131 Neer Neer 9.4.3 Tendinosis 131 of the acromion ( 1972). Thus it was who 9.4.4 Partial Thickness Tears 133 introduced the concept of an impingement syndrome 9.4.5 Full-Thickness Tears 134 continuum ranging from chronic bursitis and partial 9.4.5.1 Subacromial Distance 136 tears to complete tears of the supraspinatus tendon, 9.4.5.2 Peribursal Fat Plane 137 which may extend to involve other parts of the cuff 9.4.5.3 Intramuscular Cysts 137 Neer Matsen 9.4.6 Massive Tears 137 ( 1972; 1990). -

Pes Anserine Bursitis
BRIGHAM AND WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: Pes Anserine Bursitis ICD 9 Codes: 726.61 Case Type / Diagnosis: The pes anserine bursa lies behind the medial hamstring, which is composed of the tendons of the sartorius, gracilis and semitendinosus (SGT) muscles. Because these 3 tendons splay out on the anterior aspect of the tibia and give the appearance of the foot of a goose, pes anserine bursitis is also known as goosefoot bursitis.1 These muscles provide for medial stabilization of the knee by acting as a restraint to excessive valgus opening. They also provide a counter-rotary torque function to the knee joint. The pes anserine has an eccentric role during the screw-home mechanism that dampens the effect of excessively forceful lateral rotation that may accompany terminal knee extension.2 Pes anserine bursitis presents as pain, tenderness and swelling over the anteromedial aspect of the knee, 4 to 5 cm below the joint line.3 Pain increases with knee flexion, exercise and/or stair climbing. Inflammation of this bursa is common in overweight, middle-aged women, and may be associated with osteoarthritis of the knee. It also occurs in athletes engaged in activities such as running, basketball, and racquet sports.3 Other risk factors include: 1 • Incorrect training techniques, or changes in terrain and/or distanced run • Lack of flexibility in hamstring muscles • Lack of knee extension • Patellar malalignment Indications for Treatment: • Knee Pain • Knee edema • Decreased active and /or passive ROM of lower extremities • Biomechanical dysfunction lower extremities • Muscle imbalances • Impaired muscle performance (focal weakness or general conditioning) • Impaired function Contraindications: • Patients with active signs/symptoms of infection (fever, chills, prolonged and obvious redness or swelling at hip joint). -

Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment
Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment The glenohumeral joint is the most mobile joint in the human body, but this same characteristic also makes it the least stable joint.1-3 The rotator cuff is a group of muscles that are important in supporting the glenohumeral joint, essential in almost every type of shoulder movement.4 These muscles maintain dynamic joint stability which not only avoids mechanical obstruction but also increases the functional range of motion at the joint.1,2 However, dysfunction of these stabilizers often leads to a complex pattern of degeneration, rotator cuff tear arthropathy that often involves subacromial impingement.2,22 Rotator cuff tear arthropathy is strikingly prevalent and is the most common cause of shoulder pain and dysfunction.3,4 It appears to be age-dependent, affecting 9.7% of patients aged 20 years and younger and increasing to 62% of patients of 80 years and older ( P < .001); odds ratio, 15; 95% CI, 9.6-24; P < .001.4 Etiology for rotator cuff pathology varies but rotator cuff tears and tendinopathy are most common in athletes and the elderly.12 It can be the result of a traumatic event or activity-based deterioration such as from excessive use of arms overhead, but some argue that deterioration of these stabilizers is part of the natural aging process given the trend of increased deterioration even in individuals who do not regularly perform overhead activities.2,4 The factors affecting the rotator cuff and subsequent treatment are wide-ranging. The major objectives of this exposition are to describe rotator cuff anatomy, biomechanics, and subacromial impingement; expound upon diagnosis and assessment; and discuss surgical and conservative interventions. -

Gluteal Tendinopathy
Gluteal Tendinopathy What is a Gluteal Tendinopathy? In lying Up until recently hip bursitis was diagnosed as the main Either on your bad hip or with bad cause of lateral hip pain but recent studies suggest that an hip hanging across body like so irritation of the gluteus muscle tendon is the likeliest cause. The tendon attaches onto a bony prominence (greater trochanter) and it is here that the tendon is subject to All these positions lead to increase friction of the tendon, compressive forces leading to irritation. can cause pain and slow the healing process. This can result in pain over the lateral hip which can refer down the outside For sleeping you might like to try these positions: of the thigh and into the knee. How common is it? Gluteal tendinopathy is relatively common affecting 10-25% of the population. It is 3 times more prevalent in women than men and is most common in women between the ages of 40 and 60. One of the reasons for this is women It is also important to modify your activity. Avoid or reduce tend to have a greater angle at their hip joint increasing things that flare up your pain, this could be climbing stairs compressive forces on the tendon. or hills or those longer walks/runs. Signs and Symptoms Exercise Therapy • Pain on the outside of your hip, can refer down outside of the thigh to the knee This is best administered by a Physiotherapist to suit the • Worse when going up and/or down stairs individual but below is a rough guide to exercises which • Worse lying on affected side (and sometimes on the can help a gluteal tendinopathy. -
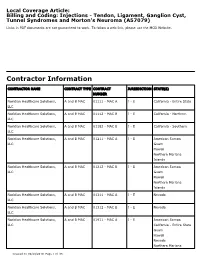
Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079)
Local Coverage Article: Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, A and B MAC 01111 - MAC A J - E California - Entire State LLC Noridian Healthcare Solutions, A and B MAC 01112 - MAC B J - E California - Northern LLC Noridian Healthcare Solutions, A and B MAC 01182 - MAC B J - E California - Southern LLC Noridian Healthcare Solutions, A and B MAC 01211 - MAC A J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01212 - MAC B J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01311 - MAC A J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01312 - MAC B J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01911 - MAC A J - E American Samoa LLC California - Entire State Guam Hawaii Nevada Northern Mariana Created on 09/28/2019. Page 1 of 33 CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Islands Article Information General Information Original Effective Date 10/01/2019 Article ID Revision Effective Date A57079 N/A Article Title Revision Ending Date Billing and Coding: Injections - Tendon, Ligament, N/A Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma Retirement Date N/A Article Type Billing and Coding AMA CPT / ADA CDT / AHA NUBC Copyright Statement CPT codes, descriptions and other data only are copyright 2018 American Medical Association. -

The Anatomy of the Deep Infrapatellar Bursa of the Knee Robert F
0363-5465/98/2626-0129$02.00/0 THE AMERICAN JOURNAL OF SPORTS MEDICINE, Vol. 26, No. 1 © 1998 American Orthopaedic Society for Sports Medicine The Anatomy of the Deep Infrapatellar Bursa of the Knee Robert F. LaPrade,* MD Department of Orthopaedic Surgery, University of Minnesota, Minneapolis, Minnesota ABSTRACT knee joint, and to define a consistent surgical approach to the deep infrapatellar bursa. Disorders of the deep infrapatellar bursa are important to include in the differential diagnosis of anterior knee pain. Knowledge regarding its anatomic location can MATERIALS AND METHODS aid the clinician in establishing a proper diagnosis. Fifty cadaveric knees were dissected, and the deep infrapa- Thorough dissections of the anterior aspect of the knee of tellar bursa had a consistent anatomic location in all 50 nonpaired cadaveric knees were performed. There were specimens. The deep infrapatellar bursa was located 27 male and 23 female cadaveric knees with 25 right and directly posterior to the distal 38% of the patellar ten- 25 left knees. The average age of the specimens was 71.8 don, just proximal to its insertion on the tibial tubercle. years (range, 42 to 93). After the skin and subcutaneous There was no communication to the knee joint. Its tissues of the anterior aspect of the knee were carefully average width at the most proximal margin of the tibial dissected away, an approach to the deep infrapatellar tubercle was slightly wider than the average distal bursa of the knee was made through medial and lateral width of the patellar tendon. It was found to be partially arthrotomy incisions along the patella, followed by compartmentalized, with a fat pad apron extending transection of the quadriceps tendon from the patella. -

IGHS Poster 01: History of the Australian Hand Surgery Society
IGHS Poster 01: History of the Australian Hand Surgery Society Category: Other Keyword: Other Not a clinical study ♦ Michael Tonkin, MD ♦ Richard Honner, MD Hypothesis: The Australian Hand Club was established in 1972, following discussion between members of the New South Wales Hand Surgery Association and the plastic surgeons of Melbourne under the direction of Sir Benjamin Rank, who became the first President. The other elected Office Bearers were: President Elect - Alan McJannet Secretary - Frank Harvey Treasurer - Richard Honner Committee Members - Peter Millroy, Don Robinson, Bernard O’Brien In 1990 the name was changed to the Australian Hand Surgery Society. This now has 159 active members, 18 overseas members, 28 honorary members and 9 provisional members. The current Board consists of: President - Randall Sach President Elect - David Stabler Ex-officio President - Stephen Coleman Secretary - Philip Griffin Treasurer - Douglass Wheen Executive Committee - Anthony Beard, David McCombe, Jeffrey Ecker An Annual Scientific Meeting with overseas Guest Professors is conducted each year, often associated with a separate two day program in hand surgery for Registrars on surgical training schemes in Australia and New Zealand. The AHSS also convenes hand surgery programmes for the Annual Scientific Meetings of the Australian Orthopaedic Association and the Royal Australasian College of Surgeons. Combined meetings with other hand surgery societies have been held, including with New Zealand, Singapore and most recently with the ASSH in Kauai, USA, March 2012. The AHSS became a member of the International Federation of Societies for Surgery of the Hand (IFSSH) in 1977 and was a founding member of the Asia-Pacific Federation of Societies for Surgery of the Hand (APFSSH) in 1997.