Bioarchaeological Implications of Calcaneal Spurs in the Medieval Nubian Population of Kulubnarti
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Evaluation of the Inferior Calcaneal Spurs Influence on Plantar Fascia
Evaluation of the Inferior Calcaneal Spurs Influence on Plantar Fascia Thickness Clint Jiroux, PMS-II ; Kyle Schwickerath, PMS-II; Frank Felix, PMS-II; Chad Smith, PMS-II; Matt Greenblatt, PMS-II Arizona School of Podiatric Medicine – Midwestern University Printing: This poster is 48” wide by 36” high. It’s STATEMENT OF PURPOSE ANALYSIS & DISCUSSION designed to be printed on a large • Plantar fasciitis is a common pathology associated with plantar heel pain. It is Through ultrasound, our research revealed the spur does not show a reported that in the United States, two million patients are treated for plantar relationship to the thickness of the plantar fascia. Thus, based on our data, printer. fasciitis annually, and accounts for 15% of all foot disorders (1). A frequent diagnostic measurements of the fascia band thickness should not take into association with plantar fasciitis is the presence of an inferior calcaneal heel consideration the presence of a heel spur. This data is supportive to the spur. Often debated by medical professionals; Is the heel spur an incidental or consensus of 4mm being the diagnostic value for plantar fasciitis. Additionally, causation of plantar fasciitis (2-4)? Using ultrasound imaging, the plantar fascia this strengthens support of the heel spur being an incidental finding with plantar Figure 1. Type A inferior calcaneal spur that extends above the plantar fascia. (A) Type B inferior calcaneal Figure 2. Longitudinal ultrasound of the plantar fascia with the presence of an inferior calcaneal spur. (A) A fasciitis. thickness can be quantifiably measured to determine plantar fasciitis. An spur that extends into the plantar fascia. -

The Painful Heel Comparative Study in Rheumatoid Arthritis, Ankylosing Spondylitis, Reiter's Syndrome, and Generalized Osteoarthrosis
Ann Rheum Dis: first published as 10.1136/ard.36.4.343 on 1 August 1977. Downloaded from Annals of the Rheumatic Diseases, 1977, 36, 343-348 The painful heel Comparative study in rheumatoid arthritis, ankylosing spondylitis, Reiter's syndrome, and generalized osteoarthrosis J. C. GERSTER, T. L. VISCHER, A. BENNANI, AND G. H. FALLET From the Department of Medicine, Division of Rheumatology, University Hospital, Geneva, Switzerland SUMMARY This study presents the frequency of severe and mild talalgias in unselected, consecutive patients with rheumatoid arthritis, ankylosing spondylitis, Reiter's syndrome, and generalized osteoarthosis. Achilles tendinitis and plantar fasciitis caused a severe talalgia and they were observed mainly in males with Reiter's syndrome or ankylosing spondylitis. On the other hand, sub-Achilles bursitis more frequently affected women with rheumatoid arthritis and rarely gave rise to severe talalgias. The simple calcaneal spur was associated with generalized osteoarthrosis and its frequency increased with age. This condition was not related to talalgias. Finally, clinical and radiological involvement of the subtalar and midtarsal joints were observed mainly in rheumatoid arthritis and occasionally caused apes valgoplanus. copyright. A 'painful heel' syndrome occurs at times in patients psoriasis, urethritis, conjunctivitis, or enterocolitis. with inflammatory rheumatic disease or osteo- The antigen HLA B27 was present in 29 patients arthrosis, causing significant clinical problems. Very (80%O). few studies have investigated the frequency and characteristics of this syndrome. Therefore we have RS 16 PATIENTS studied unselected groups of patients with rheuma- All of our patients had the complete triad (non- toid arthritis (RA), ankylosing spondylitis (AS), gonococcal urethritis, arthritis, and conjunctivitis). -

9 Impingement and Rotator Cuff Disease
Impingement and Rotator Cuff Disease 121 9 Impingement and Rotator Cuff Disease A. Stäbler CONTENTS Shoulder pain and chronic reduced function are fre- quently heard complaints in an orthopaedic outpa- 9.1 Defi nition of Impingement Syndrome 122 tient department. The symptoms are often related to 9.2 Stages of Impingement 123 the unique anatomic relationships present around the 9.3 Imaging of Impingement Syndrome: Uri Imaging Modalities 123 glenohumeral joint ( 1997). Impingement of the 9.3.1 Radiography 123 rotator cuff and adjacent bursa between the humeral 9.3.2 Ultrasound 126 head and the coracoacromial arch are among the most 9.3.3 Arthrography 126 common causes of shoulder pain. Neer noted that 9.3.4 Magnetic Resonance Imaging 127 elevation of the arm, particularly in internal rotation, 9.3.4.1 Sequences 127 9.3.4.2 Gadolinium 128 causes the critical area of the cuff to pass under the 9.3.4.3 MR Arthrography 128 coracoacromial arch. In cadaver dissections he found 9.4 Imaging Findings in Impingement Syndrome alterations attributable to mechanical impingement and Rotator Cuff Tears 130 including a ridge of proliferative spurs and excres- 9.4.1 Bursal Effusion 130 cences on the undersurface of the anterior margin 9.4.2 Imaging Following Impingement Test Injection 131 Neer Neer 9.4.3 Tendinosis 131 of the acromion ( 1972). Thus it was who 9.4.4 Partial Thickness Tears 133 introduced the concept of an impingement syndrome 9.4.5 Full-Thickness Tears 134 continuum ranging from chronic bursitis and partial 9.4.5.1 Subacromial Distance 136 tears to complete tears of the supraspinatus tendon, 9.4.5.2 Peribursal Fat Plane 137 which may extend to involve other parts of the cuff 9.4.5.3 Intramuscular Cysts 137 Neer Matsen 9.4.6 Massive Tears 137 ( 1972; 1990). -

Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment
Rotator Cuff and Subacromial Impingement Syndrome: Anatomy, Etiology, Screening, and Treatment The glenohumeral joint is the most mobile joint in the human body, but this same characteristic also makes it the least stable joint.1-3 The rotator cuff is a group of muscles that are important in supporting the glenohumeral joint, essential in almost every type of shoulder movement.4 These muscles maintain dynamic joint stability which not only avoids mechanical obstruction but also increases the functional range of motion at the joint.1,2 However, dysfunction of these stabilizers often leads to a complex pattern of degeneration, rotator cuff tear arthropathy that often involves subacromial impingement.2,22 Rotator cuff tear arthropathy is strikingly prevalent and is the most common cause of shoulder pain and dysfunction.3,4 It appears to be age-dependent, affecting 9.7% of patients aged 20 years and younger and increasing to 62% of patients of 80 years and older ( P < .001); odds ratio, 15; 95% CI, 9.6-24; P < .001.4 Etiology for rotator cuff pathology varies but rotator cuff tears and tendinopathy are most common in athletes and the elderly.12 It can be the result of a traumatic event or activity-based deterioration such as from excessive use of arms overhead, but some argue that deterioration of these stabilizers is part of the natural aging process given the trend of increased deterioration even in individuals who do not regularly perform overhead activities.2,4 The factors affecting the rotator cuff and subsequent treatment are wide-ranging. The major objectives of this exposition are to describe rotator cuff anatomy, biomechanics, and subacromial impingement; expound upon diagnosis and assessment; and discuss surgical and conservative interventions. -
Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079)
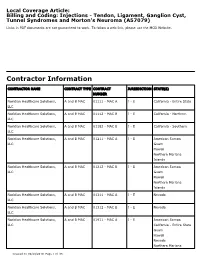
Local Coverage Article: Billing and Coding: Injections - Tendon, Ligament, Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma (A57079) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Noridian Healthcare Solutions, A and B MAC 01111 - MAC A J - E California - Entire State LLC Noridian Healthcare Solutions, A and B MAC 01112 - MAC B J - E California - Northern LLC Noridian Healthcare Solutions, A and B MAC 01182 - MAC B J - E California - Southern LLC Noridian Healthcare Solutions, A and B MAC 01211 - MAC A J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01212 - MAC B J - E American Samoa LLC Guam Hawaii Northern Mariana Islands Noridian Healthcare Solutions, A and B MAC 01311 - MAC A J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01312 - MAC B J - E Nevada LLC Noridian Healthcare Solutions, A and B MAC 01911 - MAC A J - E American Samoa LLC California - Entire State Guam Hawaii Nevada Northern Mariana Created on 09/28/2019. Page 1 of 33 CONTRACTOR NAME CONTRACT TYPE CONTRACT JURISDICTION STATE(S) NUMBER Islands Article Information General Information Original Effective Date 10/01/2019 Article ID Revision Effective Date A57079 N/A Article Title Revision Ending Date Billing and Coding: Injections - Tendon, Ligament, N/A Ganglion Cyst, Tunnel Syndromes and Morton's Neuroma Retirement Date N/A Article Type Billing and Coding AMA CPT / ADA CDT / AHA NUBC Copyright Statement CPT codes, descriptions and other data only are copyright 2018 American Medical Association. -

IGHS Poster 01: History of the Australian Hand Surgery Society
IGHS Poster 01: History of the Australian Hand Surgery Society Category: Other Keyword: Other Not a clinical study ♦ Michael Tonkin, MD ♦ Richard Honner, MD Hypothesis: The Australian Hand Club was established in 1972, following discussion between members of the New South Wales Hand Surgery Association and the plastic surgeons of Melbourne under the direction of Sir Benjamin Rank, who became the first President. The other elected Office Bearers were: President Elect - Alan McJannet Secretary - Frank Harvey Treasurer - Richard Honner Committee Members - Peter Millroy, Don Robinson, Bernard O’Brien In 1990 the name was changed to the Australian Hand Surgery Society. This now has 159 active members, 18 overseas members, 28 honorary members and 9 provisional members. The current Board consists of: President - Randall Sach President Elect - David Stabler Ex-officio President - Stephen Coleman Secretary - Philip Griffin Treasurer - Douglass Wheen Executive Committee - Anthony Beard, David McCombe, Jeffrey Ecker An Annual Scientific Meeting with overseas Guest Professors is conducted each year, often associated with a separate two day program in hand surgery for Registrars on surgical training schemes in Australia and New Zealand. The AHSS also convenes hand surgery programmes for the Annual Scientific Meetings of the Australian Orthopaedic Association and the Royal Australasian College of Surgeons. Combined meetings with other hand surgery societies have been held, including with New Zealand, Singapore and most recently with the ASSH in Kauai, USA, March 2012. The AHSS became a member of the International Federation of Societies for Surgery of the Hand (IFSSH) in 1977 and was a founding member of the Asia-Pacific Federation of Societies for Surgery of the Hand (APFSSH) in 1997. -

Haglund's Syndrome, Retrocalaneal Exostosis
Open Access Review Article DOI: 10.7759/cureus.820 Haglund’s Syndrome: A Commonly Seen Mysterious Condition Raju Vaishya 1 , Amit Kumar Agarwal 1 , Ahmad Tariq Azizi 2 , Vipul Vijay 1 1. Orthopaedics, Indraprastha Apollo Hospitals 2. Orthopaedics, Herat Regional Hospital, Herat, Afghanistan Corresponding author: Amit Kumar Agarwal, [email protected] Abstract Haglund’s deformity was first described by Patrick Haglund in 1927. It is also known as retrocalcaneal exostosis, Mulholland deformity, and ‘pump bump.' It is a very common clinical condition, but still poorly understood. Haglund’s deformity is an abnormality of the bone and soft tissues in the foot. An enlargement of the bony section of the heel (where the Achilles tendon is inserted) triggers this condition. The soft tissue near the back of the heel can become irritated when the large, bony lump rubs against rigid shoes. The aetiology is not well known, but some probable causes like a tight Achilles tendon, a high arch of the foot, and heredity have been suggested as causes. Middle age is the most common age of affection, females are more affected than males, and the occurence is often bilateral. A clinical feature of this condition is pain in the back of the heel, which is more after rest. Clinical evaluation and lateral radiographs of the ankle are mostly enough to make a diagnosis of Haglund’s syndrome. Haglund’s syndrome is often treated conservatively by altering the heel height in shoe wear, orthosis, physiotherapy, and anti-inflammatory drugs. Surgical excision of the bony exostoses of the calcaneum is only required in resistant cases. -

Musculoskeletal Diagnostic Imaging
Musculoskeletal Diagnostic Imaging Vivek Kalia, MD MPH October 02, 2019 Course: Sports Medicine for the Primary Care Physician Department of Radiology University of Michigan @VivekKaliaMD [email protected] Objectives • To review anatomy of joints which commonly present for evaluation in the primary care setting • To review basic clinical features of particular musculoskeletal conditions affecting these joints • To review key imaging features of particular musculoskeletal conditions affecting these joints Outline • Joints – Shoulder – Hip • Rotator Cuff Tendinosis / • Osteoarthritis Tendinitis • (Greater) Trochanteric bursitis • Rotator Cuff Tears • Hip Abductor (Gluteal Tendon) • Adhesive Capsulitis (Frozen Tears Shoulder) • Hamstrings Tendinosis / Tears – Elbow – Knee • Lateral Epicondylitis • Osteoarthritis • Medical Epicondylitis • Popliteal / Baker’s cyst – Hand/Wrist • Meniscus Tear • Rheumatoid Arthritis • Ligament Tear • Osteoarthritis • Cartilage Wear Outline • Joints – Ankle/Foot • Osteoarthritis • Plantar Fasciitis • Spine – Degenerative Disc Disease – Wedge Compression Deformity / Fracture Shoulder Shoulder Rotator Cuff Tendinosis / Tendinitis • Rotator cuff comprised of 4 muscles/tendons: – Supraspinatus – Infraspinatus – Teres minor – Subscapularis • Theory of rotator cuff degeneration / tearing with time: – Degenerative partial-thickness tears allow superior migration of the humeral head in turn causes abrasion of the rotator cuff tendons against the undersurface of the acromion full-thickness tears may progress to -

ESSR 2013 | 1 2 | ESSR 2013 Essrsport 2013 Injuries Musculoskeletal Radiology June 13–15, MARBELLA/SPAIN
Final Programme property of Marbella City Council ESSRSport 2013 Injuries MUSCULOSKELETAL RADIOLOGY JUNE 13–15, MARBELLA/SPAIN ESSRSport 2013 Injuries MUSCULOSKELETAL RADIOLOGY JUNE 13–15, MARBELLA/SPAIN Content 3 Welcome 4–5 ESSR Committee & Invited Speakers 6 General Information 11/13 Programme Overview ESSR 2013 | 1 2 | ESSR 2013 ESSRSport 2013 Injuries MUSCULOSKELETAL RADIOLOGY JUNE 13–15, MARBELLA/SPAIN from the ESSR 2013 Congress President ME Welcome LCO On behalf of the ESSR it is a pleasure to invite you to participate in the 20th Annual Scientific Meeting WE of the European Skeletal Society to be held in Marbella, Spain, on June 13–15, 2013, at the Palacio de Congresos located in the center of the city. The scientific programme will focus on “Sports Lesions”, with a refresher course lasting two days dedicated to actualised topics. The programme will include focus sessions and hot topics, as well as different sessions of the subcommittees of the society. There will be a special session on Interventional Strategies in Sports Injuries. The popular “hands on” ultrasound workshops in MSK ultrasound will be held on Thursday 13, 2013, during the afternoon, with the topic of Sports Lesions. Basic and advanced levels will be offered. A state-of-the-art technical exhibition will display the most advanced technical developments in the area of musculoskeletal pathology. The main lobby will be available for workstations for the EPOS as well as technical exhibits. Marbella is located in the south of Spain, full of life and with plenty of cultural and tourist interest, with architectural treasures of the traditional and popular Andalusian culture. -

Open Fracture As a Rare Complication of Olecranon Enthesophyte in a Patient with Gout Rafid Kakel, MD, and Joseph Tumilty, MD
A Case Report & Literature Review Open Fracture as a Rare Complication of Olecranon Enthesophyte in a Patient With Gout Rafid Kakel, MD, and Joseph Tumilty, MD has been reported in the English literature. The patient Abstract provided written informed consent for print and elec- Enthesophytes are analogous to osteophytes of osteo- tronic publication of this case report. arthritis. Enthesopathy is the pathologic change of the enthesis, the insertion site of tendons, ligaments, and CASE REPORT joint capsules on the bone. In gout, the crystals of mono- A 50-year-old man with chronic gout being treated with sodium urate monohydrate may provoke an inflamma- tory reaction that eventually may lead to ossification at allopurinol (and indomethacin on an as-needed basis) those sites (enthesophytes). Here we report the case of presented to the emergency department. He reported a man with chronic gout who sustained an open fracture of an olecranon enthesophyte when he fell on his left elbow. To our knowledge, no other case of open fracture of an enthesophyte has been reported in the English literature. nthesophytes are analogous to osteophytes of osteoarthritis. Enthesopathy is the pathologic change of the enthesis, the insertion site of ten- dons, ligaments, and joint capsules on the bone. EEnthesopathy occurs in a wide range of conditions, notably spondyloarthritides, crystal-induced diseases, and repeated minor trauma to the tendinous attach- ments to bones. Enthesopathy can be asymptomatic or symptomatic. In gout, crystals of monosodium urate are found in and around the joints—the cartilage, epiphyses, synovial membrane, tendons, ligaments, and enthesis. Figure 1. Small wound at patient’s left elbow. -

Clinical and Imaging Assessment of Peripheral Enthesitis in Ankylosing Spondylitis
Special RepoRt Clinical and imaging assessment of peripheral enthesitis in ankylosing spondylitis Enthesitis, defined as inflammation of the origin and insertion of ligaments, tendons, aponeuroses, annulus fibrosis and joint capsules, is a hallmark of ankylosing spondylitis. The concept of entheseal organ prone to pathological changes in ankylosing spondylitis and other spondyloarthritis is well recognized. The relevant role of peripheral enthesitis is supported by the evidence that this feature, on clinical examination, has been included in the classification criteria of Amor (heel pain or other well-defined enthesopathic pain), European Spondiloarthropathy Study Group and Assessment in SpondyloArthritis International Society for axial and peripheral spondyloarthritis. Nevertheless, the assessment of enthesitis has been improved by imaging techniques to carefully detect morphological abnormalities and to monitor disease activity. 1 Keywords: ankylosing spondylitis n clinical assessment n enthesitis n MrI Antonio Spadaro* , n spondyloarthritis n ultrasound Fabio Massimo Perrotta1, In primary ankylosing spondylitis (AS) the fre- has proven to be a highly sensitive and nonin- Alessia Carboni1 quency of peripheral enthesitis has been found vasive tool to assess the presence of enthesitis, & Antongiulio Scarno1 to be between 25 and 58% [1], however, the real characterized by hypoechogenicity with loss 1Dipartimento di Medicina Interna e prevalence of this feature depends on the type of tendon fibrillar pattern, tendon thickening, Specialità -

12-1098 ) Issued: September 19, 2012 U.S
United States Department of Labor Employees’ Compensation Appeals Board __________________________________________ ) S.R., Appellant ) ) and ) Docket No. 12-1098 ) Issued: September 19, 2012 U.S. POSTAL SERVICE, POST OFFICE, ) Harrisburg, PA, Employer ) __________________________________________ ) Appearances: Case Submitted on the Record Appellant, pro se Office of Solicitor, for the Director DECISION AND ORDER Before: RICHARD J. DASCHBACH, Chief Judge ALEC J. KOROMILAS, Alternate Judge MICHAEL E. GROOM, Alternate Judge JURISDICTION On April 23, 2012 appellant filed a timely appeal from a December 15, 2011 merit decision of the Office of Workers’ Compensation Programs (OWCP) and an April 6, 2012 nonmerit decision denying his request for reconsideration. Pursuant to the Federal Employees’ Compensation Act1 (FECA) and 20 C.F.R. §§ 501.2(c) and 501.3, the Board has jurisdiction over the merits of this case. ISSUES The issues are: (1) whether appellant met his burden of proof to establish that he sustained a right foot injury in the performance of duty on February 3, 2011; and (2) whether OWCP properly denied his request for further merit review under 5 U.S.C. § 8128(a). 1 5 U.S.C. § 8101 et seq. FACTUAL HISTORY On February 23, 2011 appellant, then a 60-year-old letter carrier, filed a traumatic injury claim (Form CA-1) alleging that on February 3, 2011 he sustained a right foot and heel injury when he was walking his route and felt pain. He notified his supervisor on February 23, 2011. In medical reports dated February 8 to March 30, 2011, appellant’s attending physician, Dr. Marc Frankel, a podiatrist, reported that appellant complained of right heel foot pain, worse when he would step down, plantar medial, poststatic dyskinesia.