Therapies for Psoriatic Enthesopathy. a Systematic Review CHRISTOPHER T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juvenile Spondyloarthropathies: Inflammation in Disguise
PP.qxd:06/15-2 Ped Perspectives 7/25/08 10:49 AM Page 2 APEDIATRIC Volume 17, Number 2 2008 Juvenile Spondyloarthropathieserspective Inflammation in DisguiseP by Evren Akin, M.D. The spondyloarthropathies are a group of inflammatory conditions that involve the spine (sacroiliitis and spondylitis), joints (asymmetric peripheral Case Study arthropathy) and tendons (enthesopathy). The clinical subsets of spondyloarthropathies constitute a wide spectrum, including: • Ankylosing spondylitis What does spondyloarthropathy • Psoriatic arthritis look like in a child? • Reactive arthritis • Inflammatory bowel disease associated with arthritis A 12-year-old boy is actively involved in sports. • Undifferentiated sacroiliitis When his right toe starts to hurt, overuse injury is Depending on the subtype, extra-articular manifestations might involve the eyes, thought to be the cause. The right toe eventually skin, lungs, gastrointestinal tract and heart. The most commonly accepted swells up, and he is referred to a rheumatologist to classification criteria for spondyloarthropathies are from the European evaluate for possible gout. Over the next few Spondyloarthropathy Study Group (ESSG). See Table 1. weeks, his right knee begins hurting as well. At the rheumatologist’s office, arthritis of the right second The juvenile spondyloarthropathies — which are the focus of this article — toe and the right knee is noted. Family history is might be defined as any spondyloarthropathy subtype that is diagnosed before remarkable for back stiffness in the father, which is age 17. It should be noted, however, that adult and juvenile spondyloar- reported as “due to sports participation.” thropathies exist on a continuum. In other words, many children diagnosed with a type of juvenile spondyloarthropathy will eventually fulfill criteria for Antinuclear antibody (ANA) and rheumatoid factor adult spondyloarthropathy. -

Atraumatic Bilateral Achilles Tendon Rupture: an Association of Systemic
378 Kotnis, Halstead, Hormbrey Acute compartment syndrome may be a of the body of gastrocnemius has been result of any trauma to the limb. The trauma is reported in athletes.7 8 This, however, is the J Accid Emerg Med: first published as 10.1136/emj.16.5.378 on 1 September 1999. Downloaded from usually a result of an open or closed fracture of first reported case of acute compartment the bones, or a crush injury to the limb. Other syndrome caused by a gastrocnemius muscle causes include haematoma, gun shot or stab rupture in a non-athlete. wounds, animal or insect bites, post-ischaemic swelling, vascular damage, electrical injuries, burns, prolonged tourniquet times, etc. Other Conclusion causes of compartment syndrome are genetic, Soft tissue injuries and muscle tears occur fre- iatrogenic, or acquired coagulopathies, infec- quently in athletes. Most injuries result from tion, nephrotic syndrome or any cause of direct trauma. Indirect trauma resulting in decreased tissue osmolarity and capillary per- muscle tears and ruptures can cause acute meability. compartment syndrome in athletes. It is also Chronic compartment syndrome is most important to keep in mind the possibility of typically an exercise induced condition charac- similar injuries in a non-athlete as well. More terised by a relative inadequacy of musculofas- research is needed to define optimal manage- cial compartment size producing chronic or ment patterns and potential strategies for recurring pain and/or disability. It is seen in injury prevention. athletes, who often have recurring leg pain that Conflict of interest: none. starts after they have been exercising for some Funding: none. -

Juvenile Spondyloarthritis / Enthesitis Related Arthritis (Spa-ERA) Version of 2016
https://www.printo.it/pediatric-rheumatology/GB/intro Juvenile Spondyloarthritis / Enthesitis Related Arthritis (SpA-ERA) Version of 2016 1. WHAT IS JUVENILE SPONDYLOARTHRITIS/ENTHESITIS- RELATED ARTHRITIS (SpA-ERA) 1.1 What is it? Juvenile SpA-ERA constitutes a group of chronic inflammatory diseases of the joints (arthritis), as well as tendon and ligament attachments to certain bones (enthesitis) and affects predominantly the lower limbs and in some cases the pelvic and spinal joints (sacroiliitis - buttock pain and spondylitis - back pain). Juvenile SpA-ERA is significantly more common in people that have a positive blood test for the genetic factor HLA-B27. HLA-B27 is a protein located on the surface of immune cells. Remarkably, only a fraction of people with HLA-B27 ever develops arthritis. Thus, the presence of HLA-B27 is not enough to explain the development of the disease. To date, the exact role of HLA-B27 in the origin of the disease remains unknown. However, it is known that in very few cases the onset of arthritis is preceded by gastrointestinal or urogenital infection (known as reactive arthritis). Juvenile SpA-ERA is closely related to the spondyloarthritis with onset in adulthood and most researchers believe these diseases share the same origin and characteristics. Most children and adolescents with juvenile spondyloarthritis would be diagnosed as affected by ERA and even psoriatic arthritis. It is important that the names "juvenile spondyloarthritis", "enthesitis-related arthritis" and in some cases "psoriatic arthritis" may be the same from a clinical and therapeutic point of view. 1 / 12 1.2 What diseases are called juvenile SpA-ERA? As mentioned above, juvenile spondyloarthritis is the name for a group of diseases; the clinical features may overlap with each other, including axial and peripheral spondyloarthritis, ankylosing spondylitis, undifferentiated spondyloarthritis, psoriatic arthritis, reactive arthritis and arthritis associated with Crohn’s disease and ulcerative colitis. -

The Correlations Between Dimensions of the Normal Tendon And
www.nature.com/scientificreports OPEN The correlations between dimensions of the normal tendon and tendinopathy changed Achilles tendon in routine magnetic resonance imaging Pawel Szaro 1,2,3* & Khaldun Ghali Gataa2 This comparative study aimed to investigate how tendinopathy-related lesions change correlations in the dimensions of the Achilles tendon. Our experimental group included 74 patients. The mean age was 52.9 ± 10.4 years. The control group included 81 patients with a mean age was 35.2 ± 13.6 years, p < .001. The most signifcant diference in correlation was the thickness of the tendon and the midportion’s width, which was more signifcant in the tendinopathy (r = .49 vs. r = .01, p < .001). The correlation was positive between width and length of the insertion but negative in normal tendons (r = .21 vs. r = − .23, p < .001). The correlation was between the midportions width in tendinopathy and the tendon’s length but negative in the normal tendon (r = .16 vs. r = − .23, p < .001). The average thickness of the midportion in tendinopathy was 11.2 ± 3.3 mm, and 4.9 ± 0.5 mm in the control group, p < .001. The average width of the midportion and insertion was more extensive in the experimental group, 17.2 ± 3.1 mm vs. 14.7 ± 1.8 mm for the midportion and 31.0 ± 3.9 mm vs. 25.7 ± 3.0 mm for insertion, respectively, p < .001. The tendon’s average length was longer in tendinopathy (83.5 ± 19.3 mm vs. 61.5 ± 14.4 mm, p < .001). The dimensions correlations in normal Achilles tendon and tendinopathic tendon difer signifcantly. -
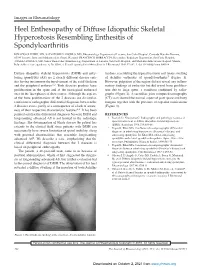
Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis
Images in Rheumatology Heel Enthesopathy of Diffuse Idiopathic Skeletal Hyperostosis Resembling Enthesitis of Spondyloarthritis IGNAZIO OLIVIERI, MD, SALVATORE D’ANGELO, MD, Rheumatology Department of Lucania, San Carlo Hospital, Contrada Macchia Romana, 85100 Potenza, Italy; and Madonna delle Grazie Hospital; FRANCESCO BORRACCIA, Researcher, Radiology Department, San Carlo Hospital; ANGELA PADULA, MD, Senior Researcher, Rheumatology Department of Lucania, San Carlo Hospital, and Madonna delle Grazie Hospital, Matera, Italy. Address correspondence to Dr. Olivieri; E-mail: [email protected]. J Rheumatol 2010;37:192–3; doi.10.3899/jrheum.090514 Diffuse idiopathic skeletal hyperostosis (DISH) and anky- tendons, resembling the typical fusiform soft tissue swelling losing spondylitis (AS) are 2 clearly different disease enti- of Achilles enthesitis of spondyloarthritis5 (Figure 1). ties having in common the involvement of the axial skeleton However, palpation of the region did not reveal any inflam- and the peripheral entheses1,2. Both diseases produce bone matory findings of enthesitis but did reveal bone prolifera- proliferation in the spine and at the extraspinal entheseal tion due to large spurs, a condition confirmed by radio- sites in the later phases of their course. Although the aspects graphs (Figure 2). A sacroiliac joint computed tomography of the bone proliferations of the 2 diseases are dissimilar, (CT) scan showed the normal aspect of joint space and bony confusion of radiographic differential diagnosis between the margins together with the presence of capsular ossifications 2 diseases exists, partly as a consequence of a lack of aware- (Figure 3). ness of their respective characteristic features2,3. It has been pointed out that the differential diagnosis between DISH and REFERENCES longstanding advanced AS is not limited to the radiologic 1. -

Clinical and Imaging Assessment of Peripheral Enthesitis in Ankylosing Spondylitis
Special RepoRt Clinical and imaging assessment of peripheral enthesitis in ankylosing spondylitis Enthesitis, defined as inflammation of the origin and insertion of ligaments, tendons, aponeuroses, annulus fibrosis and joint capsules, is a hallmark of ankylosing spondylitis. The concept of entheseal organ prone to pathological changes in ankylosing spondylitis and other spondyloarthritis is well recognized. The relevant role of peripheral enthesitis is supported by the evidence that this feature, on clinical examination, has been included in the classification criteria of Amor (heel pain or other well-defined enthesopathic pain), European Spondiloarthropathy Study Group and Assessment in SpondyloArthritis International Society for axial and peripheral spondyloarthritis. Nevertheless, the assessment of enthesitis has been improved by imaging techniques to carefully detect morphological abnormalities and to monitor disease activity. 1 Keywords: ankylosing spondylitis n clinical assessment n enthesitis n MrI Antonio Spadaro* , n spondyloarthritis n ultrasound Fabio Massimo Perrotta1, In primary ankylosing spondylitis (AS) the fre- has proven to be a highly sensitive and nonin- Alessia Carboni1 quency of peripheral enthesitis has been found vasive tool to assess the presence of enthesitis, & Antongiulio Scarno1 to be between 25 and 58% [1], however, the real characterized by hypoechogenicity with loss 1Dipartimento di Medicina Interna e prevalence of this feature depends on the type of tendon fibrillar pattern, tendon thickening, Specialità -

Foot Pain & Psoriatic Arthritis
WHEATON • ROCKVILLE • CHEVY CHASE • WASHINGTON, DC Foot Pain & Psoriatic Arthritis Daniel El-Bogdadi, MD, FACR Arthritis and Rheumatism Associates, P.C. Do you feel heel pain in the Plantar fasciitis ARTHRITIS morning or after a period of can be caused by inactivity? This might be an a number AND indication of plantar fasciitis that of factors, RHEUMATISM could be part of an underlying including aerobic ASSOCIATES, P.C. psoriatic arthritis condition. dance exercise, running, and Board Certified Rheumatologists Psoriatic arthritis is an inflammatory ballet. arthritis that typically causes pain, Herbert S.B. Baraf MD FACP MACR swelling and stiffness in the Robert L. Rosenberg peripheral joints or spine. However, MD FACR CCD if you have psoriatic arthritis, you Evan L. Siegel may notice that not only are the MD FACR joints and spine involved, but Emma DiIorio symptoms may also occur in the soft MD FACR tissue such as tendons or ligaments. David G. Borenstein MD MACP MACR This usually happens in a place Alan K. Matsumoto where tendons and ligaments attach There are several treatment options MD FACP FACR to bone, known as the enthesis. for plantar fasciitis: David P. Wolfe When this attachment gets inflamed MD FACR it is called enthesitis. • The first immediate intervention is Paul J. DeMarco MD FACP FACR to decrease or stop any Shari B. Diamond One of the more common areas to repetitive activities, such as MD FACP FACR get enthesitis is in the Achilles running or dancing, which may Ashley D. Beall tendon. Another common area for aggravate the condition. MD FACR inflammation is in thick band of (over) Angus B. -

Spondylitis Diseases Psoriasis
CD8 T-cell Spondylitis leads to the development of syndesmophytes and ankylosis Spondyloarthritis Diseases Cognitive ASp Recognition TCR •A group of autoimmune Unknown Self Antigenic Peptide diseases that in common Presented by class I MHC appear mediated by activation Target Cell of autoreactive CD8 T cells •Physical stress, inflammation and infection with specific microorganisms trigger the immune response •Primarily affect joints, skin, eyes and mucous membranes T cells invade the junction Annulus fibers are eroded, Progressive of annulus fibrosis and then replaced by fibrocartilage cartilaginous and vertebral body forming that ossifies to form a periosteal ossification •Autoantibodies such as ANA or RF are absent granulation tissue syndesmophyte. Subperiosteal forms a “bamboo spine”, (activated macrophages, T new bone formation ensues osteoporosis develops cells and fibroblasts) Spondylitis Diseases Sacroiliitis DAnkylosing spondylitis (ASp) DReiter’s syndrome (RS) / reactive arthritis (ReA) DPsoriatic arthritis (PsA) Undifferentiated spondyloarthritis (USpA) Enteropathic arthritis (ulcerative colitis, regional enteritis) The subchondral regions of the The cartilage on the iliac side is eroded synarthrotic SI joints are first, causing bone plate blurring, joint invaded by T cells leading to space “widening” and reactive sclerosis. Psoriasis the formulation of granulation Ultimately the resultant fibrous ankylosis tissue is replaced by bone, obliterating the SI joint Spondyloarthritis Diseases-features common to all Enthesitis (enthesopathy) the central inflammatory unit of spondyloarthritis Entheses are the specialized fibrocartilagenous region of bone 1. Clinical:- Affect joints, skin, eyes and mucous membranes in where ligaments, tendons, fascia or joint capsules insert varying proportions with characteristic joint involvement: Spondylitis (inflammation of vertebral discs), sacroiliitis (sacroiliac joints) and enthesitis (tendon insertions). -

Assessment of Enthesitis in Psoriatic Arthritis
Editorial Assessment of Enthesitis in Psoriatic Arthritis There has been an increasing focus on enthesitis in psoriatic They found a higher prevalence of entheseal tenderness in arthritis (PsA). Enthesitis, defined as inflammation at the FM but more enthesitis using US in PsA and psoriasis. insertion of tendons and ligaments into bone, has been Overall, B-mode US changes were common, particularly proposed as the primary pathological lesion of PsA, and this around the knee, with power Doppler abnormalities being hypothesis has received support from animal models that less frequent across the 3 groups of patients. Therefore, is have focused on the enthesis in spondyloarthropathy-like my advice to clinicians to rely only on the Achilles tendon 1,2 disease . Enthesitis is part of the entry “stem” for the justified by this report? No, because clinically an equal ClASsification for Psoriatic ARthritis criteria (CASPAR) proportion of patients with PsA and FM had tenderness at criteria, although it must be emphasized that only a few cases the Achilles entheseal insertion; and yes, because power 3 of PsA had isolated entheseal involvement in that study . Doppler abnormalities at the Achilles (at cortical bone Yet the clinical evaluation of enthesitis remains a vexing insertion, pre-insertional area, and body of tendon) were problem. When delivering educational symposia, I am often found much more frequently in PsA. asked by dermatology and rheumatology colleagues how to What are the strengths and weaknesses of this study? The assess and treat enthesitis. To the dermatologists I say look authors correctly state that because none of the patients with only at the Achilles insertion because (1) it is readily identi- PsA were taking disease-modifying drugs or steroids, it is fiable, (2) it is the major enthesis of the body, and (3) likely that they represent a milder spectrum of disease, and involvement is quite specific for spondyloarthropathy (SpA). -

Achilles Tendon Disorders
BMJ 2013;346:f1262 doi: 10.1136/bmj.f1262 (Published 12 March 2013) Page 1 of 7 Clinical Review CLINICAL REVIEW Achilles tendon disorders 1 Chad A Asplund director of military sports medicine , Thomas M Best professor and pomerene chair 2 1Department of Family Medicine, Eisenhower Army Medical Center, Fort Gordon, GA 30905, USA; 2Division of Sports Medicine, Department of Family Medicine, Ohio State University, Columbus, OH 43221, USA Disorders of the Achilles tendon are common in active fasciculi from cutaneous nerves, especially the sural nerve.4 The people—competitive and recreational athletes alike—but they paratenon is a highly vascular structure, and along with the can occur in less active people. As the largest tendon in the surrounding muscle complex supplies blood to the Achilles body, the Achilles experiences repetitive strain from running, tendon.5 jumping, and sudden acceleration or deceleration, so is Cadaveric studies suggest that there is an area 2-6 cm above the susceptible to rupture and degenerative changes. This review calcaneal insertion with a relatively poor blood supply, and that aims to describe the anatomy and diagnostic evaluation of the this predisposes the region to chronic inflammation and Achilles tendon, and to discuss the best available evidence to rupture.5 6 However, in vivo studies have failed to demonstrate help in the management of Achilles tendon disorders. this “watershed” area. Direct measurement of forces reveal What are Achilles tendon disorders? loading in the Achilles tendon to be as high as 9 kilonewtons 1 (up to 12.5 times body weight) during running, which probably The Achilles tendon is the strongest tendon in the body, serving contributes to its high rate of injury.7 both the gastrocnemius and soleus muscles. -

Postural Stability and the Relationship with Enthesitis in Ankylosing Spondylitis: a Cross-Sectional Study
Available online at www.medicinescience.org Medicine Science ORIGINAL RESEARCH International Medical Journal Medicine Science 2019;8(3):613-9 Postural stability and the relationship with enthesitis in ankylosing spondylitis: A cross-sectional study Sena Tolu1, Aylin Rezvani1, Nurbanu Hindioglu1, Ibrahim Ethem Kirez1, Merve Calkin Korkmaz2 1Medipol University Faculty of Medicine, Department of Physical Medicine and Rehabilitation, Istanbul, Turkey 2Alsancak Nevvar Salih Isgoren State Hospital, Department of Physical Medicine and Rehabilitation, Izmir, Turkey Received 19 January 2019; Accepted 10 March 2019 Available online 04.04.2019 with doi:10.5455/medscience.2019.08.9030 Copyright © 2019 by authors and Medicine Science Publishing Inc. Abstract This study aims to investigate the static and dynamic postural stability in ankylosing spondylitis (AS), and to evaluate their relationships with clinical parameters, postural measurements, and enthesitis. Sixty-four patients with AS (37 males, 27 females; mean age 41.06±8.89 years) and 42 healthy individuals (23 males, 19 females; mean age 38.45±8.46 years) were involved in the study. The static and dynamic postural stability of all participants were assessed using the Biodex Balance System. Patients’ posture was evaluated using chest expansion, tragus-wall distance, and the modified Schober’s test. The disease activity (Bath Ankylosing Spondylitis Disease Activity Index: BASDAI), functional status (Bath Ankylosing Spondylitis Functional Index: BASFI), spinal mobility (Bath Ankylosing Spondylitis Metrology Index: BASMI), enthesitis score (Maastricht Ankylosing Spondylitis Enthesitis Score: MASES), pain (Visual Analogue Scale: VAS), quality of life (Ankylosing Spondylitis Quality of Life: ASQoL) and depression (Beck Depression Inventory: BDI) of all patients were evaluated. Similar demographic data were found in both groups. -

Relationships Between Ultrasound Enthesitis, Disease Activity and Axial Radiographic Structural Changes in Patients with Early S
Spondyloarthritis RMD Open: first published as 10.1136/rmdopen-2017-000482 on 7 September 2017. Downloaded from ORIGINAL articLE Relationships between ultrasound enthesitis, disease activity and axial radiographic structural changes in patients with early spondyloarthritis: data from DESIR cohort Adeline Ruyssen-Witrand,1,2,3 Bénédicte Jamard,1 Alain Cantagrel,1,3,4 Delphine Nigon,1 Damien Loeuille,5 Yannick Degboe,4 Arnaud Constantin1,3,4 To cite: Ruyssen-Witrand A, ABSTRACT Jamard B, Cantagrel A, Background To search for association between Key messages et al. Relationships between ultrasound (US) enthesis abnormalities and disease activity, ultrasound enthesitis, disease spine and sacro-iliac joints (SIJ) MRI inflammatory lesions What is already known about this subject? activity and axial radiographic and spine structural changes in a cohort of patients ► The association between disease activity and structural changes in patients suspected for axial spondyloarthritis (SpA). enthesis ultrasound findings is not clear in the with early spondyloarthritis: data literature. from DESIR cohort. RMD Open Methods Patients: Of 708 patients included in the 2017;3:e000482. doi:10.1136/ DESIR(Devenir des Spondyloarthrites Indifférenciées What does this study add? Récentes) cohort, 402 had an US enthesis assessment rmdopen-2017-000482 ► Ultrasound peripheral enthesitis was not associated and were selected for this study. Imaging: Achilles, lateral with clinical disease activity in patients with early epicondyles, superior patellar ligament, inferior patellar ► Prepublication history and axial spondyloarthritis (SpA). ligament entheses were systematically US scanned additional material is available. ► Ultrasound peripheral enthesitis was not associated To view, please visit the journal and abnormalities were summed in US structural and with sacroiliitis nor with MRI spine inflammatory online (http:// dx.