ABSTRACT the First Through Fifth Instars of the Gypsy Moth Were Tested for Development to Adults on 326 Species of Dicotyledonous Plants in Laboratory Feeding Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromfield Garden Plant List - 2009
BROMFIELD GARDEN PLANT LIST - 2009 BOTANICAL NAME COMMON NAME Acer circinatum vine maple Achillea millefolium yarrow Achillea millefolium 'Judity' yarrow 'Judity' Achillea millefolium 'La Luna' yarrow 'La Luna' Achillea millefolium 'Paprika' yarrow 'Paprika' Achillea millefolium 'Salmon' yarrow 'Salmon' Achillea millefolium 'Sonoma Coast' yarrow 'Sonoma Coast' Aesculus californica California buckeye Aquilegia formosa western columbine Arctostaphylos 'Pacific Mist' manzanita 'Pacific Mist' Arctostaphylos hookeri 'Ken Taylor' manzanita 'Ken Taylor' Aristolochia californica California pipevine Armeria maritima sea pink Artemisia pycnocephala sandhill sage Asarum caudatum wild ginger Aster chilensis California aster Aster chilensis dwarf California aster Baccharis pilularis 'Twin Peaks' dwarf coyote brush 'Twin Peaks' Berberis aquifolium var repens creeping Oregon-grape Berberis nervosa dwarf Oregon-grape Blechnum spicant deer fern Calycanthus occidentalis spice bush Camissonia cheiranthifolia beach evening primrose Carex tumulicola Berkeley sedge Carpenteria californica bush anenome Ceanothus 'Concha' wild lilac 'Concha' Ceanothus 'Tilden Park' wild lilac 'Tilden Park' Cercis occidentalis western redbud Cercocarpus betuloides mountain mahogany Clematis lasiantha chaparral clematis Cornus sericea creek dogwood Corylus cornuta western hazelnut Dicentra formosa western bleeding heart Dichondra donneliana pony's foot Dryopteris arguta coastal wood fern Dudleya caespitosa sea lettuce Dudleya farinosa bluff lettuce Dudleya pulverulenta chalk liveforever -

Survey for Special-Status Vascular Plant Species
SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES For the proposed Eagle Canyon Fish Passage Project Tehama and Shasta Counties, California Prepared for: Tehama Environmental Solutions 910 Main Street, Suite D Red Bluff, California 96080 Prepared by: Dittes & Guardino Consulting P.O. Box 6 Los Molinos, California 96055 (530) 384-1774 [email protected] Eagle Canyon Fish Passage Improvement Project - Botany Report Sept. 12, 2018 Prepared by: Dittes & Guardino Consulting 1 SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES Eagle Canyon Fish Passage Project Shasta & Tehama Counties, California T30N, R1W, SE 1/4 Sec. 25, SE1/4 Sec. 24, NE ¼ Sec. 36 of the Shingletown 7.5’ USGS Topographic Quadrangle TABLE OF CONTENTS I. Executive Summary ................................................................................................................................................. 4 II. Introduction ............................................................................................................................................................ 4 III. Project Description ............................................................................................................................................... 4 IV. Location .................................................................................................................................................................. 5 V. Methods .................................................................................................................................................................. -
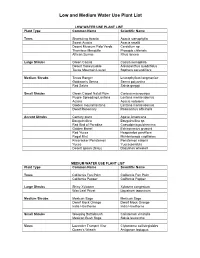
Low and Medium Water Use Plant List
Low and Medium Water Use Plant List LOW WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees Shoestring Acacia Acacia stenophylla Sweet Acacia Acacia smallii Desert Museum Palo Verde Cercidium sp. Thornless Mesquite Prosopis chilensis African Sumac Rhus lancea Large Shrubs Green Cassia Cassia nemophila Desert Honeysuckle Anisacanthus quadrifidus Texas Mountain Laurel Sophora secundiflora Medium Shrubs Texas Ranger Leucophyllum langmaniae Goldman’s Senna Senna polyantha Red Salvia Salvia greggii Small Shrubs Green Carpet Natal Plum Carissa macrocarpa Purple Spreading Lantana Lantana montevidensis Acacia Acacia redolens Golden mound lantana Lantana montevidensis Dwarf Rosemary Rosmarinus officinalis Accent Shrubs Century plant Agave Americana Bougainvillea Bougainvillea sp. Red Bird of Paradise Caesalpinia pulcherrima Golden Barrel Echinocactus grusonii Red Yucca Hesperaloe parviflora Regal Mist Muhlenbergia capillaries Firecracker Penstemon Penstemon eatonii Yucca Yucca pendula Desert spoon (Grey) Dasylirion wheeleri MEDIUM WATER USE PLANT LIST Plant Type Common Name Scientific Name Trees California Fan Palm California Fan Palm California Pepper California Pepper Large Shrubs Shiny Xylosma Xylosma congestum Wax Leaf Privet Ligustrum japonicum Medium Shrubs Mexican Sage Mexican Sage Dwarf Mock Orange Dwarf Mock Orange India Hawthorne India Hawthorne Small Shrubs Weeping Bottlebrush Calistemon viminalis Mexican Bush Sage Salvia leucantha Vines Lavender Trumpet Vine Clytostoma callistegioides Queen’s Wreath Antigonon leptopus . -

Placer Vineyards Specific Plan Placer County, California
Placer Vineyards Specific Plan Placer County, California Appendix B: Recommended Plant List Amended January 2015 Approved July 2007 R mECOm ENDED PlANt liSt APPENDIX B: RECOMMENDED PLANT LIST The list of plants below are recommended for use in Placer Vineyards within the design of its open space areas, landscape buffer corridors, streetscapes, gateways and parks. Plants similar to those listed in the table may also be substituted at the discretion of the County. OPEN SPACE Botanical Name Common Name Distribution Percentage Upland-Savanna TREES Aesculus californica California Buckeye 15% Quercus douglasii Blue Oak 15% Quercus lobata Valley Oak 40% Quercus wislizenii Interior Live Oak 15% Umbellularia california California Laurel 15% 100% SHRUBS Arctostaphylos sp Manzanita 15% Artemisia californica California Sagebrush 10% Ceanothus gloriosus Point Reyes Creeper 30% Ceanothus sp. California Lilac 10% Heteromeles arbutifolia Toyon 20% Rhamnus ilicifolia Hollyleaf Redberry 15% 100% GROUNDCOVER Bromus carinatus California Brome 15% Hordeum brachyantherum Meadow Barley 15% Muhlenbergia rigens Deergrass 40% Nassella pulchra Purple Needlegrass 15% Lupinus polyphyllus Blue Lupine 15% 100% January 2015 Placer Vineyards Specific Plan B-1 R mECOm ENDED PlANt liSt OPEN SPACE Botanical Name Common Name Distribution Percentage Riparian Woodland (2- to 5-year event creek flow) TREES Acer negundo Boxelder 5% Alnus rhombifolia White Alder 5% Fraxinus latifolia Oregon Ash 10% Populus fremontii Fremont Cottonwood 25% Quercus lobata Valley Oak 5% Salix gooddingii -

Plants That Provide Seeds and Berries
Native Plants that Provide Seeds and Berries Abies amabilis Pacific Silver Fir An attractive conifer with short dark green needles. Tolerant of shade. Squirrels and other rodents extract seeds from the large cones. Abies grandis Grand Fir Abies grandis is a tall, straight tree with short, dense branches. Grouse, nuthatches, chickadees, grosbeaks, finches, crossbills feed on the fir seeds. Sapsuckers and woodpeckers feed on the foliage. Pine white butterfly larvae eat the leaves. Acer circinatum Vine Maple Tall, erect, multi-trunked shrub or small tree with sprawling branches. Birds that eat the seeds include grosbeaks, woodpeckers, nuthatches, finches, quail, and grouse. A larvae plant for the brown tissue moth and the Polyphemus moth. A good nectar source for bees. Deer, mountain beavers, and other beavers eat the twigs and wood. Acer macrophyllum Big-leaf Maple A tree with a large, often multi-stemmed trunk and a loose, broad crown of large leaves. The rotting limbs provide a food source for insect-eating birds such as grouse, grosbeaks, kinglets, siskins, vireos, warblers, sapsuckers, woodpeckers, nuthatches, song sparrows, finches, and quail. Acer macrophyllum is a good nectar source for swallowtail butterfly larvae and bees. Deer, muskrats, and beaver eat the wood and twigs. Achillea millefolium Yarrow Aromatic herb with delicate fern-like leaves and flat-topped clusters of white flowers. Arbutus menziesii Madrone An attractive broadleaf evergreen with a twisting reddish trunk and irregular branches with an overall rounded outline. The fruit is eaten by band-tailed pigeons, quail, flickers, varied thrushes, waxwings, evening grosbeaks, mourning doves, and robins. The flowers are pollinated by spring azure butterflies and bees. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Untangling Phylogenetic Patterns and Taxonomic Confusion in Tribe Caryophylleae (Caryophyllaceae) with Special Focus on Generic
TAXON 67 (1) • February 2018: 83–112 Madhani & al. • Phylogeny and taxonomy of Caryophylleae (Caryophyllaceae) Untangling phylogenetic patterns and taxonomic confusion in tribe Caryophylleae (Caryophyllaceae) with special focus on generic boundaries Hossein Madhani,1 Richard Rabeler,2 Atefeh Pirani,3 Bengt Oxelman,4 Guenther Heubl5 & Shahin Zarre1 1 Department of Plant Science, Center of Excellence in Phylogeny of Living Organisms, School of Biology, College of Science, University of Tehran, P.O. Box 14155-6455, Tehran, Iran 2 University of Michigan Herbarium-EEB, 3600 Varsity Drive, Ann Arbor, Michigan 48108-2228, U.S.A. 3 Department of Biology, Faculty of Sciences, Ferdowsi University of Mashhad, P.O. Box 91775-1436, Mashhad, Iran 4 Department of Biological and Environmental Sciences, University of Gothenburg, Box 461, 40530 Göteborg, Sweden 5 Biodiversity Research – Systematic Botany, Department of Biology I, Ludwig-Maximilians-Universität München, Menzinger Str. 67, 80638 München, Germany; and GeoBio Center LMU Author for correspondence: Shahin Zarre, [email protected] DOI https://doi.org/10.12705/671.6 Abstract Assigning correct names to taxa is a challenging goal in the taxonomy of many groups within the Caryophyllaceae. This challenge is most serious in tribe Caryophylleae since the supposed genera seem to be highly artificial, and the available morphological evidence cannot effectively be used for delimitation and exact determination of taxa. The main goal of the present study was to re-assess the monophyly of the genera currently recognized in this tribe using molecular phylogenetic data. We used the sequences of nuclear ribosomal internal transcribed spacer (ITS) and the chloroplast gene rps16 for 135 and 94 accessions, respectively, representing all 16 genera currently recognized in the tribe Caryophylleae, with a rich sampling of Gypsophila as one of the most heterogeneous groups in the tribe. -

University of California Santa Cruz Responding to An
UNIVERSITY OF CALIFORNIA SANTA CRUZ RESPONDING TO AN EMERGENT PLANT PEST-PATHOGEN COMPLEX ACROSS SOCIAL-ECOLOGICAL SCALES A dissertation submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in ENVIRONMENTAL STUDIES with an emphasis in ECOLOGY AND EVOLUTIONARY BIOLOGY by Shannon Colleen Lynch December 2020 The Dissertation of Shannon Colleen Lynch is approved: Professor Gregory S. Gilbert, chair Professor Stacy M. Philpott Professor Andrew Szasz Professor Ingrid M. Parker Quentin Williams Acting Vice Provost and Dean of Graduate Studies Copyright © by Shannon Colleen Lynch 2020 TABLE OF CONTENTS List of Tables iv List of Figures vii Abstract x Dedication xiii Acknowledgements xiv Chapter 1 – Introduction 1 References 10 Chapter 2 – Host Evolutionary Relationships Explain 12 Tree Mortality Caused by a Generalist Pest– Pathogen Complex References 38 Chapter 3 – Microbiome Variation Across a 66 Phylogeographic Range of Tree Hosts Affected by an Emergent Pest–Pathogen Complex References 110 Chapter 4 – On Collaborative Governance: Building Consensus on 180 Priorities to Manage Invasive Species Through Collective Action References 243 iii LIST OF TABLES Chapter 2 Table I Insect vectors and corresponding fungal pathogens causing 47 Fusarium dieback on tree hosts in California, Israel, and South Africa. Table II Phylogenetic signal for each host type measured by D statistic. 48 Table SI Native range and infested distribution of tree and shrub FD- 49 ISHB host species. Chapter 3 Table I Study site attributes. 124 Table II Mean and median richness of microbiota in wood samples 128 collected from FD-ISHB host trees. Table III Fungal endophyte-Fusarium in vitro interaction outcomes. -

Site Assessemnt (PDF)
Site Assessment Report Scotts Valley Hotel SCOTTS VALLEY, SANTA CRUZ COUNTY, CALIFORNIA December 29, 2014 Prepared by: On behalf of: Johnson Marigot Consulting, LLC City Ventures, LLC Cameron Johnson Mr. Jason Bernstein 88 North Hill Drive, Suite C 444 Spear Street, Suite 200 Brisbane, California 94005 San Francisco, California 94105 1 Table Of Contents SECTION 1: Environmental Setting ................................................................................... 4 A. Project Location ........................................................................................................................... 4 B. Surrounding Land Use ................................................................................................................ 4 C. Study Area Topography and Hydrology ............................................................................... 4 D. Study Area Soil .............................................................................................................................. 5 E. Vegetation Types .......................................................................................................................... 5 SECTION 2: Methods ............................................................................................................... 7 A. Site Visit .......................................................................................................................................... 7 B. Study Limits .................................................................................................................................. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0263516 A1 CYR (43) Pub
US 20090263516A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0263516 A1 CYR (43) Pub. Date: Oct. 22, 2009 (54) PLANT EXTRACT COMPOSITION AND Publication Classification THEIR USE TO MODULATE CELLULAR (51) Int. Cl. ACTIVITY A636/8962 (2006.01) A636/00 (2006.01) (75) Inventor: Benoit CYR, St. Augustin de A6IP35/00 (2006.01) Desmaures (CA) CI2N 5/06 (2006.01) Correspondence Address: A6IR 36/3 (2006.01) SHEPPARD, MULLIN, RICHTER & HAMPTON A 6LX 36/899 (2006.01) LLP (52) U.S. Cl. ......... 424/754; 424/725; 435/375; 424/774; 990 Marsh Road 424/779; 424/755; 424/750; 424/777 Menlo Park, CA 94025 (US) (57) ABSTRACT (73) Assignee: Biopharmacopae Design Extracts from plant material, or semi-purified/purified mol International Inc., Saint-Foy (CA) ecules or compounds prepared from the extracts that demon strate the ability to modulate one or more cellular activities (21) Appl. No.: 12/263,114 are provided. The extracts are capable of slowing down, inhibiting or preventing cell migration, for example, the (22) Filed: Oct. 31, 2008 migration of endothelial cells or neoplastic cells and thus, the use of the extracts to slow down, inhibit or prevent abnormal Related U.S. Application Data cell migration in an animal is also provided. Methods of selecting and preparing the plant extracts and methods of (63) Continuation of application No. 10/526,387, filed on screening the extracts to determine their ability to modulate Oct. 6, 2005, now abandoned, filed as application No. one or more cellular activity are described. The purification or PCT/CA03/01284 on Sep. -

Verdura® Native Planting
Abronia maritime Abronia maritima is a species of sand verbena known by the common name red (Coastal) sand verbena. This is a beach-adapted perennial plant native to the coastlines of southern California, including the Channel Islands, and northern Baja California. Abronia villosa Abronia villosa is a species of sand-verbena known by the common name desert (Inland) sand-verbena. It is native to the deserts of the southwestern United States and northern Mexico and the southern California and Baja coast. Adenostoma Adenostoma fasciculatum (chamise or greasewood) is a flowering plant native to fasciculatum California and northern Baja California. This shrub is one of the most widespread (Coastal/Inland) plants of the chaparral biome. Adenostoma fasciculatum is an evergreen shrub growing to 4m tall, with dry-looking stick-like branches. The leaves are small, 4– 10 mm long and 1mm broad with a pointed apex, and sprout in clusters from the branches. Arctostaphylos Arctostaphylos uva-ursi is a plant species of the genus Arctostaphylos (manzanita). uva-ursi Its common names include kinnikinnick and pinemat manzanita, and it is one of (Coastal/Inland) several related species referred to as bearberry. Arctostaphylos Arctostaphylos edmundsii, with the common name Little Sur manzanita, is a edmundsii species of manzanita. This shrub is endemic to California where it grows on the (Coastal/Inland) coastal bluffs of Monterey County. Arctostaphylos Arctostaphylos hookeri is a species of manzanita known by the common name hookeri Hooker's manzanita. Arctostaphylos hookeri is a low shrub which is variable in (Coastal/Inland) appearance and has several subspecies. The Arctostaphylos hookeri shrub is endemic to California where its native range extends from the coastal San Francisco Bay Area to the Central Coast. -

Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems
Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems Sims, L., Goheen, E., Kanaskie, A., & Hansen, E. (2015). Alder canopy dieback and damage in western Oregon riparian ecosystems. Northwest Science, 89(1), 34-46. doi:10.3955/046.089.0103 10.3955/046.089.0103 Northwest Scientific Association Version of Record http://cdss.library.oregonstate.edu/sa-termsofuse Laura Sims,1, 2 Department of Botany and Plant Pathology, Oregon State University, 1085 Cordley Hall, Corvallis, Oregon 97331 Ellen Goheen, USDA Forest Service, J. Herbert Stone Nursery, Central Point, Oregon 97502 Alan Kanaskie, Oregon Department of Forestry, 2600 State Street, Salem, Oregon 97310 and Everett Hansen, Department of Botany and Plant Pathology, 1085 Cordley Hall, Oregon State University, Corvallis, Oregon 97331 Alder Canopy Dieback and Damage in Western Oregon Riparian Ecosystems Abstract We gathered baseline data to assess alder tree damage in western Oregon riparian ecosystems. We sought to determine if Phytophthora-type cankers found in Europe or the pathogen Phytophthora alni subsp. alni, which represent a major threat to alder forests in the Pacific Northwest, were present in the study area. Damage was evaluated in 88 transects; information was recorded on damage type (pathogen, insect or wound) and damage location. We evaluated 1445 red alder (Alnus rubra), 682 white alder (Alnus rhombifolia) and 181 thinleaf alder (Alnus incana spp. tenuifolia) trees. We tested the correlation between canopy dieback and canker symptoms because canopy dieback is an important symptom of Phytophthora disease of alder in Europe. We calculated the odds that alder canopy dieback was associated with Phytophthora-type cankers or other biotic cankers.