Microorganism Identification & Typing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 10: Classification of Microorganisms
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy • 8 successive taxa are used to classify each species: Domain Kingdom Phylum Class Order Family Genus **species can also contain different strains** Species Scientific Nomenclature To avoid confusion, every type of organism must be referred to in a consistent way. The current system of nomenclature (naming) has been in use since the 18th century: • every type of organism is referred by its genus name followed by its specific epithet (i.e., species name) Homo sapiens (H. sapiens) Escherichia coli (E. coli) • name should be in italics and only the genus is capitalized which can also be abbreviated • names are Latin (or “Latinized” Greek) with the genus being a noun and the specific epithet an adjective **strain info can be listed after the specific epithet (e.g., E. coli DH5α)** 2. Methods of Identification Biochemical Testing In addition to morphological (i.e., appearance under the microscope) and differential staining characteristics, microorganisms can also be identified by their biochemical “signatures”: • the nutrient requirements and metabolic “by-products” of of a particular microorganism • different growth media can be used to test the physiological characteristics of a microorganism • e.g., medium with lactose only as energy source • e.g., medium that reveals H2S production **appearance on test medium reveals + or – result!** Commercial devices for rapid Identification Perform multiple tests simultaneously Enterotube II Such devices involve the simultaneous inoculation of various test media: • ~24 hrs later the panel of results reveals ID of organism! Use of Dichotomous Keys Series of “yes/no” biochemical tests to ID organism. -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Marine Microorganisms: Evolution and Solution to Pollution Fu L Li1, Wang B1,2
COMMENTARY Marine microorganisms: Evolution and solution to pollution Fu L Li1, Wang B1,2 Li FL, Wang B. Marine microorganisms: Evolution and solution to pollution. J Mar Microbiol. 2018;2(1):4-5. nce ocean nurtured life, now she needs our care. Marine microorganism will be an opportunity to further understand ourselves and to seek for new Ois the host of ocean in all ages. We should learn from them humbly. methods of fighting old infections. Marine microorganism is tightly bond with human during the history of evolution and nowadays’ environment pollution. Along with industrial revolution, our marine ecosystem suffered serious pollutions. Microplastics are tiny plastic particles (<5 mm) (Figure 1B), Although the topic is still in debate, life is probably originated from which poison marine lives. Because these microplastics are very hard to be submarine in hydrothermal vent systems (1). In the journey of evolution, our degraded, it is predicted that there will be more microplastics than fish in biosphere was completely dominated by microbes for a very long time (Figure ocean by the year 2050 (7). Since marine sediments are considered as the sink 1A). Human being evolves with those microorganisms. Consequently, of microplastics and marine microbes are key dwellers of marine sediments, the influences of microorganisms can be found in all aspects of human more attention should be paid on the interactions between microplastics biology. More than 65% of our genes originated with bacteria, archaea, and and marine microbes. Actually, a call for this has been published in 2011 unicellular eukaryotes, including those genes responsible for host-microbe (8). -
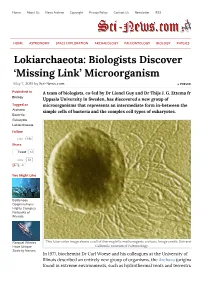
Lokiarchaeota: Biologists Discover 'Missing Link' Microorganism
Home About Us News Archive Copyright Privacy Policy Contact Us Newsletter RSS HOME ASTRONOMY SPACE EXPLORATION ARCHAEOLOGY PALEONTOLOGY BIOLOGY PHYSICS Lokiarchaeota: Biologists Discover ‘Missing Link’ Microorganism May 7, 2015 by Sci-News.com « PREVIOUS Published in A team of biologists, co-led by Dr Lionel Guy and Dr Thijs J. G. Ettema from Biology Uppsala University in Sweden, has discovered a new group of Tagged as microorganisms that represents an intermediate form in-between the Archaea simple cells of bacteria and the complex cell types of eukaryotes. Bacteria Eukaryote Lokiarchaeota Follow Like 16k Share Tweet 12 Like 58 41 You Might Like Bottlenose Dolphins Form Highly Complex Networks of Friends Rorqual Whales This false-color image shows a cell of thermophilic methanogenic archaea. Image credit: University of Have Unique California Museum of Paleontology. Stretchy Nerves In 1977, biochemist Dr Carl Woese and his colleagues at the University of Illinois described an entirely new group of organisms, the Archaea (originally found in extreme environments, such as hydrothermal vents and terrestrial hot springs). The scientists were studying relationships among the prokaryotes using DNA Extinction of sequences, and found that Archaea have distinct molecular characteristics World’s Largest separating them from bacteria as well as from eukaryotes. They proposed that Herbivores May Lead to Empty life can be divided into three domains: Eukaryota, Eubacteria, and Landscapes, Say Archaebacteria. Researchers Despite that archaeal cells were simple and small like bacteria, scientists found that Archaea were more closely related to organisms with complex cell types, a group collectively known as ‘eukaryotes.’ This observation has puzzled Sichuan Bush biologists for years. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Marine Extremophiles: a Source of Hydrolases for Biotechnological Applications
Mar. Drugs 2015, 13, 1925-1965; doi:10.3390/md13041925 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Article Marine Extremophiles: A Source of Hydrolases for Biotechnological Applications Gabriel Zamith Leal Dalmaso 1,2, Davis Ferreira 3 and Alane Beatriz Vermelho 1,* 1 BIOINOVAR—Biotechnology laboratories: Biocatalysis, Bioproducts and Bioenergy, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] 2 Graduate Program in Plant Biotechnology, Health and Science Centre, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil 3 BIOINOVAR—Biotechnology Laboratories: Virus-Cell Interaction, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-(21)-3936-6743; Fax: +55-(21)-2560-8344. Academic Editor: Kirk Gustafson Received: 1 December 2014 / Accepted: 25 March 2015 / Published: 3 April 2015 Abstract: The marine environment covers almost three quarters of the planet and is where evolution took its first steps. Extremophile microorganisms are found in several extreme marine environments, such as hydrothermal vents, hot springs, salty lakes and deep-sea floors. The ability of these microorganisms to support extremes of temperature, salinity and pressure demonstrates their great potential for biotechnological processes. Hydrolases including amylases, cellulases, peptidases and lipases from hyperthermophiles, psychrophiles, halophiles and piezophiles have been investigated for these reasons. -
L Deposits of Microorganisms and Other Biological Material L
PCT Applicant’s Guide – International Phase – Annex L Page 1 L Deposits of Microorganisms L and Other Biological Material Requirements of designated and elected Offices Only Offices whose applicable national law contains provisions concerning the deposits of microorganisms and other biological material are listed in this table. Unless otherwise indicated in the table, deposits may be made for the purposes of patent procedure before these Offices with any depositary institution having acquired the status of international depositary authority under the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure (these institutions are indicated further in this Annex and notifications related thereto may be consulted under www.wipo.int/treaties/en/registration/budapest/). Further information concerning the requirements of international depository authorities under the Budapest Treaty is available at www.wipo.int/treaties/en/registration/budapest/guide/ Time (if any) earlier than Additional indications (if any) 16 months from priority date by which must be given besides which applicant must furnish: those prescribed in Rule 13bis.3(a)(i) to (iii) Designated the indications any additional pursuant to notifications from (or elected) Office prescribed in matter specified the Offices concerned Rule 13bis.3(a)(i) in the adjacent to (iii) right-hand column AL – Albania General Directorate of None At the time of To the extent available to the Industrial Property (GDIP) filing (as part of applicant, relevant information (Albania) the application) on the characteristics of the microorganism Deposits may also be made for the purposes of patent procedure before the General Directorate of Industrial Property (GDIP) (Albania) with any depositary institution specialized for that purpose. -

Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives
marine drugs Review Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives Emiliana Tortorella 1,†, Pietro Tedesco 1,2,†, Fortunato Palma Esposito 1,3,†, Grant Garren January 1,†, Renato Fani 4, Marcel Jaspars 5 and Donatella de Pascale 1,3,* 1 Institute of Protein Biochemistry, National Research Council, I-80131 Naples, Italy; [email protected] (E.T.); [email protected] (P.T.); [email protected] (F.P.E.); [email protected] (G.G.J.) 2 Laboratoire d’Ingénierie des Systèmes Biologiques et des Procédés, INSA, 31400 Toulouse, France 3 Stazione Zoologica “Anthon Dorn”, Villa Comunale, I-80121 Naples, Italy 4 Department of Biology, University of Florence, Sesto Fiorentino, I-50019 Florence, Italy; renato.fani@unifi.it 5 Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Aberdeen, Scotland AB24 3UE, UK; [email protected] * Correspondence: [email protected]; Tel.: +39-0816-132-314; Fax: +39-0816-132-277 † These authors equally contributed to the work. Received: 23 July 2018; Accepted: 27 September 2018; Published: 29 September 2018 Abstract: The increasing emergence of new forms of multidrug resistance among human pathogenic bacteria, coupled with the consequent increase of infectious diseases, urgently requires the discovery and development of novel antimicrobial drugs with new modes of action. Most of the antibiotics currently available on the market were obtained from terrestrial organisms or derived semisynthetically from fermentation products. The isolation of microorganisms from previously unexplored habitats may lead to the discovery of lead structures with antibiotic activity. The deep-sea environment is a unique habitat, and deep-sea microorganisms, because of their adaptation to this extreme environment, have the potential to produce novel secondary metabolites with potent biological activities. -

Role of the Cell-Wall Structure in the Retention of Bacteria by Microfiltration Membranes
Open Archive Toulouse Archive Ouverte (OATAO) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is an author-deposited version published in: http://oatao.univ-toulouse.fr/ Eprints ID: 5910 To link to this article: DOI:10.1016/J.MEMSCI.2008.09.049 URL: http://dx.doi.org/10.1016/J.MEMSCI.2008.09.049 To cite this version: Lebleu, Nathalie and Roques, Christine and Aimar, Pierre and Causserand, Christel (2009) Role of the cell-wall structure in the retention of bacteria by microfiltration membranes. Journal of Membrane Science, vol. 326 (n°1). pp. 178-185. ISSN 0376-7388 Any correspondence concerning this service should be sent to the repository administrator: [email protected] Role of the cell•wall structure in the retention of bacteria by microfiltration membranes Nathalie Lebleu a, Christine Roques b, Pierre Aimar a, Christel Causserand a,∗ a Laboratoire de Génie Chimique, Université Paul Sabatier, 31062 Toulouse Cedex 9, France b LU 49, Adhésion Bactérienne et Formation de Biofilms, Université Paul Sabatier, 31062 Toulouse Cedex 9, France a b s t r a c t This experimental study investigates the retention of bacteria by porous membranes. The transfer of bacteria larger than the nominal pore size of microfiltration track•etched membranes has been studied for several kinds of bacterial strains. This unexpected transfer does not correlate to the hydrophobicity, neither to the surface charge of the microorganism, as suggested in previous reports. We conclude that, in our conditions, the kind of bacteria (Gram•positive or Gram•negative) is finally the most important parameter. -

Cell Structure and Function in the Bacteria and Archaea
4 Chapter Preview and Key Concepts 4.1 1.1 DiversityThe Beginnings among theof Microbiology Bacteria and Archaea 1.1. •The BacteriaThe are discovery classified of microorganismsinto several Cell Structure wasmajor dependent phyla. on observations made with 2. theThe microscope Archaea are currently classified into two 2. •major phyla.The emergence of experimental 4.2 Cellscience Shapes provided and Arrangements a means to test long held and Function beliefs and resolve controversies 3. Many bacterial cells have a rod, spherical, or 3. MicroInquiryspiral shape and1: Experimentation are organized into and a specific Scientificellular c arrangement. Inquiry in the Bacteria 4.31.2 AnMicroorganisms Overview to Bacterialand Disease and Transmission Archaeal 4.Cell • StructureEarly epidemiology studies suggested how diseases could be spread and 4. Bacterial and archaeal cells are organized at be controlled the cellular and molecular levels. 5. • Resistance to a disease can come and Archaea 4.4 External Cell Structures from exposure to and recovery from a mild 5.form Pili allowof (or cells a very to attach similar) to surfacesdisease or other cells. 1.3 The Classical Golden Age of Microbiology 6. Flagella provide motility. Our planet has always been in the “Age of Bacteria,” ever since the first 6. (1854-1914) 7. A glycocalyx protects against desiccation, fossils—bacteria of course—were entombed in rocks more than 3 billion 7. • The germ theory was based on the attaches cells to surfaces, and helps observations that different microorganisms years ago. On any possible, reasonable criterion, bacteria are—and always pathogens evade the immune system. have been—the dominant forms of life on Earth. -

Food Microbial Ecology - Eugenia Bezirtzoglou
MEDICAL SCIENCES - Food Microbial Ecology - Eugenia Bezirtzoglou FOOD MICROBIAL ECOLOGY Eugenia Bezirtzoglou, Democritus University of Thrace, Faculty of Agricultural Development, Department of Food Science and Technology, Laboratory of Microbiology, Biotechnology and Hygiene and Laboratory of food Processing, Orestiada, Greece Keywords: Food, Microbial Ecology Contents 1. Scope of Microbial Ecology 2. Food Microbial Ecosystem 3. Diversity of Habitat 4. Factors influencing the Growth and Survival of Microorganisms in Foods 5. Food Spoilage and its Microbiology 6. Fermented and Microbial Foods 7. Conclusions Related Chapters Glossary Bibliography Biographical Sketch Summary Microbial ecology is the study of microorganisms in their proper environment and their interactions with it. Microbial ecology can give us answers about our origin, our place in the earth ecosystem as well as on our connection to the great diversity of all other organisms. In this vein, studying microbial ecology questions should help to explain the role of microbes in the environment, in food production, in bioengineering and chemicals items and as result will improve our lives. There is a plethora of microorganisms on our planet, most microorganisms remain unknown. It is estimated that we have knowledge only of 1% of the microbial species on Earth. Multiple studies in intestinal ecology have been greatly hampered by the inaccuracy and limitations of culture methods. Many bacteria are difficult to culture or are unculturable, and often media are not truly specific or are too selective for certain bacteria. Furthermore it is impossible to study and compare complete ecosystems, as they exist in the human body, by culturing methods. Molecular tools introduced in microbial ecology made it possible to study the composition of the microecosystems in a different way, which is not dependent on culture techniques. -

Microbial Risk Assessment Guideline
EPA/100/J-12/001 USDA/FSIS/2012-001 MICROBIAL RISK ASSESSMENT GUIDELINE PATHOGENIC MICROORGANISMS WITH FOCUS ON FOOD AND WATER Prepared by the Interagency Microbiological Risk Assessment Guideline Workgroup July 2012 Microbial Risk Assessment Guideline Page ii DISCLAIMER This guideline document represents the current thinking of the workgroup on the topics addressed. It is not a regulation and does not confer any rights for or on any person and does not operate to bind USDA, EPA, any other federal agency, or the public. Further, this guideline is not intended to replace existing guidelines that are in use by agencies. The decision to apply methods and approaches in this guideline, either totally or in part, is left to the discretion of the individual department or agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Environmental Protection Agency (EPA) (2012). Microbial Risk Assessment Guideline: Pathogenic Microorganisms with Focus on Food and Water. EPA/100/J-12/001 Microbial Risk Assessment Guideline Page iii TABLE OF CONTENTS Disclaimer .......................................................................................................................... ii Interagency Workgroup Members ................................................................................ vii Preface ............................................................................................................................. viii Abbreviations ..................................................................................................................