Marine Microorganisms: Evolution and Solution to Pollution Fu L Li1, Wang B1,2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 10: Classification of Microorganisms
Chapter 10: Classification of Microorganisms 1. The Taxonomic Hierarchy 2. Methods of Identification 1. The Taxonomic Hierarchy Phylogenetic Tree of the 3 Domains Taxonomic Hierarchy • 8 successive taxa are used to classify each species: Domain Kingdom Phylum Class Order Family Genus **species can also contain different strains** Species Scientific Nomenclature To avoid confusion, every type of organism must be referred to in a consistent way. The current system of nomenclature (naming) has been in use since the 18th century: • every type of organism is referred by its genus name followed by its specific epithet (i.e., species name) Homo sapiens (H. sapiens) Escherichia coli (E. coli) • name should be in italics and only the genus is capitalized which can also be abbreviated • names are Latin (or “Latinized” Greek) with the genus being a noun and the specific epithet an adjective **strain info can be listed after the specific epithet (e.g., E. coli DH5α)** 2. Methods of Identification Biochemical Testing In addition to morphological (i.e., appearance under the microscope) and differential staining characteristics, microorganisms can also be identified by their biochemical “signatures”: • the nutrient requirements and metabolic “by-products” of of a particular microorganism • different growth media can be used to test the physiological characteristics of a microorganism • e.g., medium with lactose only as energy source • e.g., medium that reveals H2S production **appearance on test medium reveals + or – result!** Commercial devices for rapid Identification Perform multiple tests simultaneously Enterotube II Such devices involve the simultaneous inoculation of various test media: • ~24 hrs later the panel of results reveals ID of organism! Use of Dichotomous Keys Series of “yes/no” biochemical tests to ID organism. -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Recent Advances in Biocatalysts Engineering for Polyethylene Terephthalate Plastic Waste Green Recycling
Environment International 145 (2020) 106144 Contents lists available at ScienceDirect Environment International journal homepage: www.elsevier.com/locate/envint Review article Recent advances in biocatalysts engineering for polyethylene terephthalate plastic waste green recycling Nadia A. Samak a,b,c,1, Yunpu Jia a,b,1, Moustafa M. Sharshar a,b, Tingzhen Mu a, Maohua Yang a, Sumit Peh a,b, Jianmin Xing a,b,* a CAS Key Laboratory of Green Process and Engineering & State Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, PR China b College of Chemical Engineering, University of Chinese Academy of Sciences, 19 A Yuquan Road, Beijing 100049, PR China c Processes Design and Development Department, Egyptian Petroleum Research Institute, Nasr City, 11727 Cairo, Egypt ARTICLE INFO ABSTRACT Handling Editor: Guo-ping Sheng The massive waste of poly(ethylene terephthalate) (PET) that ends up in the landfills and oceans and needs hundreds of years for degradation has attracted global concern. The poor stability and productivity of the Keywords: available PET biocatalysts hinder their industrial applications. Active PET biocatalysts can provide a promising Plastic waste avenue for PET bioconversion and recycling. Therefore, there is an urgent need to develop new strategies that Poly(ethylene terephthalate) could enhance the stability, catalytic activity, solubility, productivity, and re-usability of these PET biocatalysts Recycling under harsh conditions such as high temperatures, pH, and salinity. This has raised great attention in using Biocatalysts ’ Bioengineering bioengineering strategies to improve PET biocatalysts robustness and catalytic behavior. Herein, historical and forecasting data of plastic production and disposal were critically reviewed. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
Lokiarchaeota: Biologists Discover 'Missing Link' Microorganism
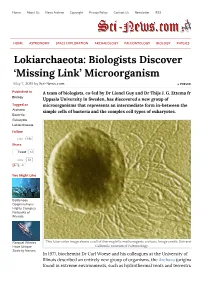
Home About Us News Archive Copyright Privacy Policy Contact Us Newsletter RSS HOME ASTRONOMY SPACE EXPLORATION ARCHAEOLOGY PALEONTOLOGY BIOLOGY PHYSICS Lokiarchaeota: Biologists Discover ‘Missing Link’ Microorganism May 7, 2015 by Sci-News.com « PREVIOUS Published in A team of biologists, co-led by Dr Lionel Guy and Dr Thijs J. G. Ettema from Biology Uppsala University in Sweden, has discovered a new group of Tagged as microorganisms that represents an intermediate form in-between the Archaea simple cells of bacteria and the complex cell types of eukaryotes. Bacteria Eukaryote Lokiarchaeota Follow Like 16k Share Tweet 12 Like 58 41 You Might Like Bottlenose Dolphins Form Highly Complex Networks of Friends Rorqual Whales This false-color image shows a cell of thermophilic methanogenic archaea. Image credit: University of Have Unique California Museum of Paleontology. Stretchy Nerves In 1977, biochemist Dr Carl Woese and his colleagues at the University of Illinois described an entirely new group of organisms, the Archaea (originally found in extreme environments, such as hydrothermal vents and terrestrial hot springs). The scientists were studying relationships among the prokaryotes using DNA Extinction of sequences, and found that Archaea have distinct molecular characteristics World’s Largest separating them from bacteria as well as from eukaryotes. They proposed that Herbivores May Lead to Empty life can be divided into three domains: Eukaryota, Eubacteria, and Landscapes, Say Archaebacteria. Researchers Despite that archaeal cells were simple and small like bacteria, scientists found that Archaea were more closely related to organisms with complex cell types, a group collectively known as ‘eukaryotes.’ This observation has puzzled Sichuan Bush biologists for years. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Marine Extremophiles: a Source of Hydrolases for Biotechnological Applications
Mar. Drugs 2015, 13, 1925-1965; doi:10.3390/md13041925 OPEN ACCESS marine drugs ISSN 1660-3397 www.mdpi.com/journal/marinedrugs Article Marine Extremophiles: A Source of Hydrolases for Biotechnological Applications Gabriel Zamith Leal Dalmaso 1,2, Davis Ferreira 3 and Alane Beatriz Vermelho 1,* 1 BIOINOVAR—Biotechnology laboratories: Biocatalysis, Bioproducts and Bioenergy, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] 2 Graduate Program in Plant Biotechnology, Health and Science Centre, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil 3 BIOINOVAR—Biotechnology Laboratories: Virus-Cell Interaction, Institute of Microbiology Paulo de Góes, Federal University of Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941-902 Rio de Janeiro, Brazil; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +55-(21)-3936-6743; Fax: +55-(21)-2560-8344. Academic Editor: Kirk Gustafson Received: 1 December 2014 / Accepted: 25 March 2015 / Published: 3 April 2015 Abstract: The marine environment covers almost three quarters of the planet and is where evolution took its first steps. Extremophile microorganisms are found in several extreme marine environments, such as hydrothermal vents, hot springs, salty lakes and deep-sea floors. The ability of these microorganisms to support extremes of temperature, salinity and pressure demonstrates their great potential for biotechnological processes. Hydrolases including amylases, cellulases, peptidases and lipases from hyperthermophiles, psychrophiles, halophiles and piezophiles have been investigated for these reasons. -
L Deposits of Microorganisms and Other Biological Material L
PCT Applicant’s Guide – International Phase – Annex L Page 1 L Deposits of Microorganisms L and Other Biological Material Requirements of designated and elected Offices Only Offices whose applicable national law contains provisions concerning the deposits of microorganisms and other biological material are listed in this table. Unless otherwise indicated in the table, deposits may be made for the purposes of patent procedure before these Offices with any depositary institution having acquired the status of international depositary authority under the Budapest Treaty on the International Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure (these institutions are indicated further in this Annex and notifications related thereto may be consulted under www.wipo.int/treaties/en/registration/budapest/). Further information concerning the requirements of international depository authorities under the Budapest Treaty is available at www.wipo.int/treaties/en/registration/budapest/guide/ Time (if any) earlier than Additional indications (if any) 16 months from priority date by which must be given besides which applicant must furnish: those prescribed in Rule 13bis.3(a)(i) to (iii) Designated the indications any additional pursuant to notifications from (or elected) Office prescribed in matter specified the Offices concerned Rule 13bis.3(a)(i) in the adjacent to (iii) right-hand column AL – Albania General Directorate of None At the time of To the extent available to the Industrial Property (GDIP) filing (as part of applicant, relevant information (Albania) the application) on the characteristics of the microorganism Deposits may also be made for the purposes of patent procedure before the General Directorate of Industrial Property (GDIP) (Albania) with any depositary institution specialized for that purpose. -

Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives
marine drugs Review Antibiotics from Deep-Sea Microorganisms: Current Discoveries and Perspectives Emiliana Tortorella 1,†, Pietro Tedesco 1,2,†, Fortunato Palma Esposito 1,3,†, Grant Garren January 1,†, Renato Fani 4, Marcel Jaspars 5 and Donatella de Pascale 1,3,* 1 Institute of Protein Biochemistry, National Research Council, I-80131 Naples, Italy; [email protected] (E.T.); [email protected] (P.T.); [email protected] (F.P.E.); [email protected] (G.G.J.) 2 Laboratoire d’Ingénierie des Systèmes Biologiques et des Procédés, INSA, 31400 Toulouse, France 3 Stazione Zoologica “Anthon Dorn”, Villa Comunale, I-80121 Naples, Italy 4 Department of Biology, University of Florence, Sesto Fiorentino, I-50019 Florence, Italy; renato.fani@unifi.it 5 Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Aberdeen, Scotland AB24 3UE, UK; [email protected] * Correspondence: [email protected]; Tel.: +39-0816-132-314; Fax: +39-0816-132-277 † These authors equally contributed to the work. Received: 23 July 2018; Accepted: 27 September 2018; Published: 29 September 2018 Abstract: The increasing emergence of new forms of multidrug resistance among human pathogenic bacteria, coupled with the consequent increase of infectious diseases, urgently requires the discovery and development of novel antimicrobial drugs with new modes of action. Most of the antibiotics currently available on the market were obtained from terrestrial organisms or derived semisynthetically from fermentation products. The isolation of microorganisms from previously unexplored habitats may lead to the discovery of lead structures with antibiotic activity. The deep-sea environment is a unique habitat, and deep-sea microorganisms, because of their adaptation to this extreme environment, have the potential to produce novel secondary metabolites with potent biological activities. -

Marine Microbial Diversity
Marine microbial diversity 27 K. S. Sobhana Marine Biodiversity Division, Central Marine Fisheries Research Institute, Kochi-682 018 Microbes were the only form of life for the first 2-3 billion and also based on iii) concentration of nutrients and required years of planetary and biological evolution. Life most likely growth substances (Oligotrophic, Mesotrophic, Eutrophic). began in the oceans and marine microorganisms are the However, interfaces tend to be hotspots of diversity and closest living descendants of the original forms of life. Early biological activity. Marine microbial habitats at interfaces marine microorganisms also helped create the conditions include the air-water, water-sediment, water-ice, and under which subsequent life developed. More than two billion host macroorganism-water interfaces. The sub-millimeter years ago, the generation of oxygen by photosynthetic marine scale of physical and chemical variability in these habitats microorganisms helped shape the chemical environment in poses a serious challenge to studying interface habitats in which plants, animals, and all other life forms have evolved. detail. The fact that variations can even occur within a few Macroscopic life and planetary habitability completely millimetres, suggests that microbial diversity encompasses depend upon the transformations mediated by complex more than the documented evidence available. Hence, microbial communities. These microscopic factories both biogeography is gaining importance as a field of study from aerobic and anaerobic are the essential catalysts for all of the microbial diversity point of interest. Due to the innately chemical reactions within the biogeochemical cycles. Their small size of the microorganisms, environmental complexity unique metabolisms allow marine microbes to carry out many plays a major role in determining diversity. -

Microbes on a Bottle: Substrate, Season and Geography Influence Community Composition of Microbes Colonizing Marine Plastic Debris
RESEARCH ARTICLE Microbes on a Bottle: Substrate, Season and Geography Influence Community Composition of Microbes Colonizing Marine Plastic Debris Sonja Oberbeckmann1,2,3☯, A. Mark Osborn1,2,4, Melissa B. Duhaime5☯* 1 Department of Biological Sciences, University of Hull, Cottingham Road, Hull HU6 7RX, United Kingdom, a11111 2 School of Life Sciences, University of Lincoln, Brayford Pool Lincoln LN6 7TS, United Kingdom, 3 Environmental Microbiology Working Group, Leibniz Institute for Baltic Sea Research, Warnemünde, Germany, 4 School of Applied Sciences, Royal Melbourne Institute of Technology University, PO Box 77, Bundoora, VIC3083, Australia, 5 Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, Michigan, United States of America ☯ These authors contributed equally to this work. * [email protected] OPEN ACCESS Citation: Oberbeckmann S, Osborn AM, Duhaime MB (2016) Microbes on a Bottle: Substrate, Season Abstract and Geography Influence Community Composition of Microbes Colonizing Marine Plastic Debris. PLoS Plastic debris pervades in our oceans and freshwater systems and the potential ecosystem- ONE 11(8): e0159289. doi:10.1371/journal. level impacts of this anthropogenic litter require urgent evaluation. Microbes readily colonize pone.0159289 aquatic plastic debris and members of these biofilm communities are speculated to include Editor: Dee A. Carter, University of Sydney, pathogenic, toxic, invasive or plastic degrading-species. The influence of plastic-colonizing AUSTRALIA microorganisms on the fate of plastic debris is largely unknown, as is the role of plastic in Received: May 11, 2015 selecting for unique microbial communities. This work aimed to characterize microbial bio- Accepted: April 26, 2016 film communities colonizing single-use poly(ethylene terephthalate) (PET) drinking bottles, determine their plastic-specificity in contrast with seawater and glass-colonizing communi- Published: August 3, 2016 ties, and identify seasonal and geographical influences on the communities. -

Structure of the Plastic-Degrading Ideonella Sakaiensis Mhetase Bound to a Substrate
ARTICLE https://doi.org/10.1038/s41467-019-09326-3 OPEN Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate Gottfried J. Palm 1, Lukas Reisky 2, Dominique Böttcher2, Henrik Müller2, Emil A.P. Michels1, Miriam C. Walczak2, Leona Berndt1, Manfred S. Weiss3, Uwe T. Bornscheuer 2 & Gert Weber 1,4 The extreme durability of polyethylene terephthalate (PET) debris has rendered it a long-term environmental burden. At the same time, current recycling efforts still lack sustainability. Two 1234567890():,; recently discovered bacterial enzymes that specifically degrade PET represent a promising solution. First, Ideonella sakaiensis PETase, a structurally well-characterized consensus α/β-hydrolase fold enzyme, converts PET to mono-(2-hydroxyethyl) terephthalate (MHET). MHETase, the second key enzyme, hydrolyzes MHET to the PET educts terephthalate and ethylene glycol. Here, we report the crystal structures of active ligand-free MHETase and MHETase bound to a nonhydrolyzable MHET analog. MHETase, which is reminiscent of feruloyl esterases, possesses a classic α/β-hydrolase domain and a lid domain conferring substrate specificity. In the light of structure-based mapping of the active site, activity assays, mutagenesis studies and a first structure-guided alteration of substrate specificity towards bis-(2-hydroxyethyl) terephthalate (BHET) reported here, we anticipate MHETase to be a valuable resource to further advance enzymatic plastic degradation. 1 Molecular Structural Biology, University of Greifswald, Felix-Hausdorff-Str. 4, 17487 Greifswald, Germany. 2 Biotechnology & Enzyme Catalysis, University of Greifswald, Felix-Hausdorff-Str. 4, 17487 Greifswald, Germany. 3 Macromolecular Crystallography, Helmholtz-Zentrum Berlin für Materialien und Energie, Albert-Einstein-Straße15, 12489 Berlin, Germany.