Gray Triggerfish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnuridae 575
click for previous page Rajiformes: Gymnuridae 575 GYMNURIDAE Butterfly rays by J.D. McEachran, TexasA&MUniversity, USA and M.R. de Carvalho, American Museum of Natural History, New York, USA iagnostic characters:Medium to large-sized stingrays (maximum disc width over 2 m).Body strongly de- Dpressed, with head, trunk, and broadly expanded pectoral fins forming rhomboid disc. Disc at least 1.5 times broad as long. Tail very slender and short (shorter than disc), distinctly demarcated from disc.Pec- toral fins continuous along sides of head, not forming subrostral lobes or cephalic fins.Eyes and spira- cles on top of head. Some species have spiracular tentacles. Snout obtuse and angular. Nasal curtains are broadly expanded and continuous across narrow isthmus in front of mouth and are smooth-edged (with rare exceptions). Mouth is slightly arched and lacks papillae on floor. Jaws bear many small teeth in bands. Cau- dal fin always absent, dorsal fin absent in all Western Central Atlantic representatives. Pectoral fins extend distinctly posterior to origin of pelvic fins. Pelvic fins are moderately laterally expanded and not divided into anterior and posterior lobes. Some species have 1 or more long, serrated spines. Tail with longitudinal folds on upper and/or lower surfaces. Skin of upper side naked in most species, but with a variable num- ber of tubercles in large individuals of others. Colour: dorsal surface grey, light green, olive, purple, or dark brown, sometimes with a reddish cast, often marked with spots or lines; ventral surface white, sometimes with a bronze or rusty cast. disc at least 1.5 times broad as long smooth nasal curtain nostril tail slender and short mouth detail of mouth Habitat, biology, and fisheries: Butterfly rays are cosmopolitan in tropical and warm-temperate waters, usu- ally inhabiting sandy and muddy bottoms in shallow coastal waters, including estuaries and river mouths. -

A Practical Handbook for Determining the Ages of Gulf of Mexico And
A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes THIRD EDITION GSMFC No. 300 NOVEMBER 2020 i Gulf States Marine Fisheries Commission Commissioners and Proxies ALABAMA Senator R.L. “Bret” Allain, II Chris Blankenship, Commissioner State Senator District 21 Alabama Department of Conservation Franklin, Louisiana and Natural Resources John Roussel Montgomery, Alabama Zachary, Louisiana Representative Chris Pringle Mobile, Alabama MISSISSIPPI Chris Nelson Joe Spraggins, Executive Director Bon Secour Fisheries, Inc. Mississippi Department of Marine Bon Secour, Alabama Resources Biloxi, Mississippi FLORIDA Read Hendon Eric Sutton, Executive Director USM/Gulf Coast Research Laboratory Florida Fish and Wildlife Ocean Springs, Mississippi Conservation Commission Tallahassee, Florida TEXAS Representative Jay Trumbull Carter Smith, Executive Director Tallahassee, Florida Texas Parks and Wildlife Department Austin, Texas LOUISIANA Doug Boyd Jack Montoucet, Secretary Boerne, Texas Louisiana Department of Wildlife and Fisheries Baton Rouge, Louisiana GSMFC Staff ASMFC Staff Mr. David M. Donaldson Mr. Bob Beal Executive Director Executive Director Mr. Steven J. VanderKooy Mr. Jeffrey Kipp IJF Program Coordinator Stock Assessment Scientist Ms. Debora McIntyre Dr. Kristen Anstead IJF Staff Assistant Fisheries Scientist ii A Practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes Third Edition Edited by Steve VanderKooy Jessica Carroll Scott Elzey Jessica Gilmore Jeffrey Kipp Gulf States Marine Fisheries Commission 2404 Government St Ocean Springs, MS 39564 and Atlantic States Marine Fisheries Commission 1050 N. Highland Street Suite 200 A-N Arlington, VA 22201 Publication Number 300 November 2020 A publication of the Gulf States Marine Fisheries Commission pursuant to National Oceanic and Atmospheric Administration Award Number NA15NMF4070076 and NA15NMF4720399. -

Sharks in Crisis: a Call to Action for the Mediterranean
REPORT 2019 SHARKS IN CRISIS: A CALL TO ACTION FOR THE MEDITERRANEAN WWF Sharks in the Mediterranean 2019 | 1 fp SECTION 1 ACKNOWLEDGEMENTS Written and edited by WWF Mediterranean Marine Initiative / Evan Jeffries (www.swim2birds.co.uk), based on data contained in: Bartolí, A., Polti, S., Niedermüller, S.K. & García, R. 2018. Sharks in the Mediterranean: A review of the literature on the current state of scientific knowledge, conservation measures and management policies and instruments. Design by Catherine Perry (www.swim2birds.co.uk) Front cover photo: Blue shark (Prionace glauca) © Joost van Uffelen / WWF References and sources are available online at www.wwfmmi.org Published in July 2019 by WWF – World Wide Fund For Nature Any reproduction in full or in part must mention the title and credit the WWF Mediterranean Marine Initiative as the copyright owner. © Text 2019 WWF. All rights reserved. Our thanks go to the following people for their invaluable comments and contributions to this report: Fabrizio Serena, Monica Barone, Adi Barash (M.E.C.O.), Ioannis Giovos (iSea), Pamela Mason (SharkLab Malta), Ali Hood (Sharktrust), Matthieu Lapinksi (AILERONS association), Sandrine Polti, Alex Bartoli, Raul Garcia, Alessandro Buzzi, Giulia Prato, Jose Luis Garcia Varas, Ayse Oruc, Danijel Kanski, Antigoni Foutsi, Théa Jacob, Sofiane Mahjoub, Sarah Fagnani, Heike Zidowitz, Philipp Kanstinger, Andy Cornish and Marco Costantini. Special acknowledgements go to WWF-Spain for funding this report. KEY CONTACTS Giuseppe Di Carlo Director WWF Mediterranean Marine Initiative Email: [email protected] Simone Niedermueller Mediterranean Shark expert Email: [email protected] Stefania Campogianni Communications manager WWF Mediterranean Marine Initiative Email: [email protected] WWF is one of the world’s largest and most respected independent conservation organizations, with more than 5 million supporters and a global network active in over 100 countries. -

Malaysia National Plan of Action for the Conservation and Management of Shark (Plan2)
MALAYSIA NATIONAL PLAN OF ACTION FOR THE CONSERVATION AND MANAGEMENT OF SHARK (PLAN2) DEPARTMENT OF FISHERIES MINISTRY OF AGRICULTURE AND AGRO-BASED INDUSTRY MALAYSIA 2014 First Printing, 2014 Copyright Department of Fisheries Malaysia, 2014 All Rights Reserved. No part of this publication may be reproduced or transmitted in any form or by any means, electronic, mechanical, including photocopy, recording, or any information storage and retrieval system, without prior permission in writing from the Department of Fisheries Malaysia. Published in Malaysia by Department of Fisheries Malaysia Ministry of Agriculture and Agro-based Industry Malaysia, Level 1-6, Wisma Tani Lot 4G2, Precinct 4, 62628 Putrajaya Malaysia Telephone No. : 603 88704000 Fax No. : 603 88891233 E-mail : [email protected] Website : http://dof.gov.my Perpustakaan Negara Malaysia Cataloguing-in-Publication Data ISBN 978-983-9819-99-1 This publication should be cited as follows: Department of Fisheries Malaysia, 2014. Malaysia National Plan of Action for the Conservation and Management of Shark (Plan 2), Ministry of Agriculture and Agro- based Industry Malaysia, Putrajaya, Malaysia. 50pp SUMMARY Malaysia has been very supportive of the International Plan of Action for Sharks (IPOA-SHARKS) developed by FAO that is to be implemented voluntarily by countries concerned. This led to the development of Malaysia’s own National Plan of Action for the Conservation and Management of Shark or NPOA-Shark (Plan 1) in 2006. The successful development of Malaysia’s second National Plan of Action for the Conservation and Management of Shark (Plan 2) is a manifestation of her renewed commitment to the continuous improvement of shark conservation and management measures in Malaysia. -

Feeding Habits of Three Batoids in the Levantine Sea (North-Eastern Mediterranean Sea) Based on Stomach Content and Isotopic Data Emre Yemi‹S‚Ken1, Manuela G
Journal of the Marine Biological Association of the United Kingdom, page 1 of 8. # Marine Biological Association of the United Kingdom, 2017 doi:10.1017/S002531541700073X Feeding habits of three Batoids in the Levantine Sea (north-eastern Mediterranean Sea) based on stomach content and isotopic data emre yemi‹s‚ken1, manuela g. forero2, persefoni megalofonou3,lu¤tfi‹ye eryilmaz1 and joan navarro2 1Department of Biology, Section of Hydrobiology, Faculty of Science, Istanbul University, Turkey, 2Department of Conservation Biology, Estacio´n Biolo´gica de Don˜ana (EBD-CSIC), Avda. Ame´rico Vespucio s/n, Sevilla 41092, Spain, 3Department of Biology, Section of Zoology Marine Biology, National and Kapodıstrıan University of Athens, Greece Understanding the diet of marine predators is essential to defining their trophic role in an ecosystem. Elasmobranchs (sharks and batoids) are considered pivotal components of marine food webs, and are often included in the top predator or mesopre- dator groups. However, in comparison with other Mediterranean areas, research focusing on marine predators inhabiting the Levantine Sea (eastern Mediterranean Sea) is very limited. Here, we examined the feeding habits (diet, trophic width and trophic position) of three endangered batoids (Gymnura altavela (Linnaeus, 1758), Raja asterias Delaroche, 1809 and Raja clavata, Linnaeus, 1758) coexisting in Iskenderun Bay (north-eastern Levantine Sea, Mediterranean Basin) by combin- ing stomach content and stable isotope analyses. The results revealed clear differences in the trophic habits between them. Stomach contents showed differences in the diet between species, showing a clear feeding preference for teleosts in the case of G. altavela and a diet composed of fish and crustaceans in the case of R. -

Class Wars: Chondrichthyes and Osteichthyes Dominance in Chesapeake Bay, 2002-2012
Class Wars: Chondrichthyes and Osteichthyes dominance in Chesapeake Bay, 2002-2012. 01 July 2013 Introduction The objective of this analysis was to demonstrate a possible changing relationship between two Classes of fishes, Osteichthyes (the bony fishes) and Chondrichthyes (the cartilaginous fishes) in Chesapeake Bay based on 11 years of monitoring. If any changes between the two Classes appeared to be significant, either statistically or anecdotally, the data were explored further in an attempt to explain the variation. The Class Osteichthyes is characterized by having a skeleton made of bone and is comprised of the majority of fish species worldwide, while the Chondrichthyes skeleton is made of cartilage and is represented by the sharks, skates, and rays (the elasmobranch fishes) and chimaeras1. Many shark species are generally categorized as apex predators, while skates and rays and some smaller sharks can be placed into the mesopredator functional group (Myers et al., 2007). By definition, mesopredators prey upon a significant array of lower trophic groups, but also serve as the prey base for apex predators. Global demand for shark and consequential shark fishing mortality, estimated at 97 million sharks in 2010 (Worm et al., 2013), is hypothesized to have contributed to the decline of these apex predators in recent years (Baum et al., 2003 and Fowler et al., 2005), which in turn is suggested to have had a cascading effect on lower trophic levels—an increase in mesopredators and subsequent decrease in the prey base (Myers et al., 2007). According to 10 years of trawl survey monitoring of Chesapeake Bay, fish species composition of catches has shown a marked change over the years (Buchheister et al., 2013). -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Fish Identification Guide Depicts More Than 50 Species of Fish Commonly Encoun- Make the Proper Identification of Every Fish Caught
he identification of different spe- Most species of fish are distinctive in appear- ance and relatively easy to identify. However, cies of fish has become an im- closely related species, such as members of the portant concern for recreational same “family” of fish, can present problems. For these species it is important to look for certain fishermen. The proliferation of T distinctive characteristics to make a positive regulations relating to minimum identification. sizes and possession limits compels fishermen to The ensuing fish identification guide depicts more than 50 species of fish commonly encoun- make the proper identification of every fish caught. tered in Virginia waters. In addition to color illustrations of each species, the description of each species lists the distinctive characteristics which enable a positive identification. Total Length FIRST DORSAL FIN Fork Length SECOND NUCHAL DORSAL FIN BAND SQUARE TAIL NARES FORKED TAIL GILL COVER (Operculum) CAUDAL LATRAL PEDUNCLE CHIN BARBELS LINE PECTORAL CAUDAL FIN ANAL FINS FIN PELVIC FINS GILL RAKERS GILL ARCH UNDERSIDE OF GILL COVER GILL RAKER GILL FILAMENTS GILL FILAMENTS DEFINITIONS Anal Fin – The fin on the bottom of fish located between GILL ARCHES 1st the anal vent (hole) and the tail. 2nd 3rd Barbels – Slender strands extending from the chins of 4th some fish (often appearing similar to whiskers) which per- form a sensory function. Caudal Fin – The tail fin of fish. Nuchal Band – A dark band extending from behind or Caudal Peduncle – The narrow portion of a fish’s body near the eye of a fish across the back of the neck toward immediately in front of the tail. -

Sharks, Skates, Rays, and Chimaeras
SHARKS, SKATES, RAYS, AND CHIMAERAS UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES Circular 228 TABLE 1. -- tiximum sizes of camnon species of sharks Species Traditional Mucimum length Muimum length maximum size (measure<l--U. S. coa.ts) (recorde<l--world) Scientific na.rr;e from literature SixgL. st.ark .... 1 Hexanchus sp. .•..•••••••. 15 feet 5 inches 26 feet 5 inches nd hary... ..... Carcharias taurus... 10 feet 5 inches 12 feet 3 inches 15 feet 11 inches Porbeagle •....... 1 LamTUl TUlSUS........... ... 10 feet 12 feet 12 feet Sall10n shark. .... LamTUl ditropis . 8 feet 6 inches 8 feet 6 inches 12 feet L 0 .•.••.•.•.... Isurus oxyrinchus ...... ... 10 feet 6 inches 12 feet 12 feet - 13 feet 'hi te sr.ark. ..... Carcharodan carcharias. 18 feet 2 inches 21 feet 36 feet 6 inches Basking shar".... Cetorhinus maximus . 32 feet 2 inches 45 feet 40 feet - 50 feet Thresher shark... Alopias vulpinus . 18 feet 18 feet 20 feet rse shark...... Ginglymostoma cirraturn.. 9 feet 3 inches 14 feet Whale shark. ..... Rhincodan typus........ .•. 38 feet 45 feet 45 feet - 50 feet Olain dogfish.... Scyliorhinus retifer. ... .. 1 foot 5 inches 2 feet 6 inches Leopard shark.... Triakis semifasciata... 5 feet 5 feet Smooth dogfish ... Alustelus canis ......... ... 4 feet 9 inches 5 feet rieer shark...... Galeocerdo cuvieri..... ... 13 feet 10 inches 18 feet 30 feet Soupfin shark.... Galeorhinus zyopterus . .. 6 feet 5 inches 6 feet 5 inches 6 feet 5 inches Blue shark. ...... Prionace glauca ....... 11 feet 12 feet 7 inches 25 feet Bul .. shark. ...... Carcharhinus leucas. .. 9 feet 10 inches 10 feet Whi tetip shark. -

Mediterranean Sea
OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi MEDITERRANEAN The IUCN Red List of Threatened Species™ – Regional Assessment OVERVIEW OF THE CONSERVATION STATUS OF THE MARINE FISHES OF THE MEDITERRANEAN SEA Compiled by Dania Abdul Malak, Suzanne R. Livingstone, David Pollard, Beth A. Polidoro, Annabelle Cuttelod, Michel Bariche, Murat Bilecenoglu, Kent E. Carpenter, Bruce B. Collette, Patrice Francour, Menachem Goren, Mohamed Hichem Kara, Enric Massutí, Costas Papaconstantinou and Leonardo Tunesi The IUCN Red List of Threatened Species™ – Regional Assessment Compilers: Dania Abdul Malak Mediterranean Species Programme, IUCN Centre for Mediterranean Cooperation, calle Marie Curie 22, 29590 Campanillas (Parque Tecnológico de Andalucía), Málaga, Spain Suzanne R. Livingstone Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, c/o Conservation International, Arlington, VA 22202, USA David Pollard Applied Marine Conservation Ecology, 7/86 Darling Street, Balmain East, New South Wales 2041, Australia; Research Associate, Department of Ichthyology, Australian Museum, Sydney, Australia Beth A. Polidoro Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Annabelle Cuttelod Red List Unit, IUCN Species Programme, 219c Huntingdon Road, Cambridge CB3 0DL,UK Michel Bariche Biology Departement, American University of Beirut, Beirut, Lebanon Murat Bilecenoglu Department of Biology, Faculty of Arts and Sciences, Adnan Menderes University, 09010 Aydin, Turkey Kent E. Carpenter Global Marine Species Assessment, Marine Biodiversity Unit, IUCN Species Programme, Old Dominion University, Norfolk, VA 23529, USA Bruce B. -
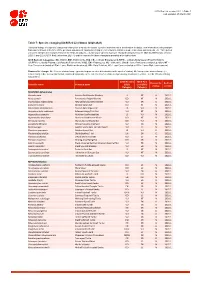
Table 7: Species Changing IUCN Red List Status (2020-2021)
IUCN Red List version 2021-1: Table 7 Last Updated: 25 March 2021 Table 7: Species changing IUCN Red List Status (2020-2021) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2020 (IUCN Red List version 2020-3) and 2021 (IUCN Red List version 2021-1) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered [CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild)], EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2020) List (2021) change version Category -

Reproductive Biology of Three Ray Species: Gymnura Micrura (Bloch
Cah. Biol. Mar. (2007) 48 : 249-257 Reproductive biology of three ray species: Gymnura micrura (Bloch & Schneider, 1801), Dasyatis guttata (Bloch & Schneider, 1801) and Dasyatis marianae Gomes, Rosa & Gadig, 2000, caught by artisanal fisheries in Northeastern Brazil Leandro YOKOTA1* and Rosângela Paula LESSA2 (1,2) Postgraduate Program in Animal Biology – Department of Zoology – Federal University of Pernambuco (UFPE) Av. Prof. Moraes Rego, 1235, Cidade Universitária, Recife – PE, Brazil. CEP 50670-420 *Corresponding author: Rua D. Pedro II, 1916, Nova Americana, Americana – SP, Brazil CEP 13466-000. E-mail: [email protected]) (2) Laboratory of Marine Population Dynamics (DIMAR) – Department of Fisheries – Federal Rural University of Pernambuco (UFRPE) Av. D. Manoel de Medeiros, s/n, Dois Irmãos, Recife – PE, Brazil. CEP 52171-900. E-mail: [email protected] Abstract: Two hundred forty-eight specimens of Gymnura micrura, 154 Dasyatis guttata and 24 Dasyatis marianae were sampled. G. micrura and D. guttata juveniles represented 78 and 88% of the sample, respectively, whereas all D. marianae individuals were adults. The maturity size of G. micrura males and females was estimated from 27 to 30 cm disk width (DW) and 34 to 36 cm DW, respectively. The maturity size of D. guttata males and females was estimated from 41 to 46 cm DW and 50 to 55 cm DW, respectively. Birth size of G. micrura was estimated between 15 and 17.4 cm DW, the one of D. guttata between12.3 and 15.3 cm DW and the one of D. marianae between 13 and 14 cm DW. Female rays appeared to mature at larger sizes than males and reached a larger maximum size.