Climatic Niche Shift and Possible Future Spread of the Invasive South African Orchid Disa Bracteata in Australia and Adjacent Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -

An Asian Orchid, Eulophia Graminea (Orchidaceae: Cymbidieae), Naturalizes in Florida
LANKESTERIANA 8(1): 5-14. 2008. AN ASIAN ORCHID, EULOPHIA GRAMINEA (ORCHIDACEAE: CYMBIDIEAE), NATURALIZES IN FLORIDA ROBE R T W. PEMBE R TON 1,3, TIMOTHY M. COLLINS 2 & SUZANNE KO P TU R 2 1Fairchild Tropical Botanic Garden, 2121 SW 28th Terrace Ft. Lauderdale, Florida 33312 2Department of Biological Sciences, Florida International University, Miami, FL 33199 3Author for correspondence: [email protected] ABST R A C T . Eulophia graminea, a terrestrial orchid native to Asia, has naturalized in southern Florida. Orchids naturalize less often than other flowering plants or ferns, butE. graminea has also recently become naturalized in Australia. Plants were found growing in five neighborhoods in Miami-Dade County, spanning 35 km from the most northern to the most southern site, and growing only in woodchip mulch at four of the sites. Plants at four sites bore flowers, and fruit were observed at two sites. Hand pollination treatments determined that the flowers are self compatible but fewer fruit were set in selfed flowers (4/10) than in out-crossed flowers (10/10). No fruit set occurred in plants isolated from pollinators, indicating that E. graminea is not autogamous. Pollinia removal was not detected at one site, but was 24.3 % at the other site evaluated for reproductive success. A total of 26 and 92 fruit were found at these two sites, where an average of 6.5 and 3.4 fruit were produced per plant. These fruits ripened and dehisced rapidly; some dehiscing while their inflorescences still bore open flowers. Fruit set averaged 9.2 and 4.5 % at the two sites. -
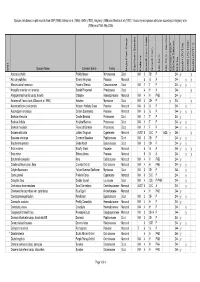
BFS244 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No beel01 (FCT28) beel02 (FCT23a) beel03 (FCT11) Wd? Acacia pulchella Prickly Moses Mimosaceae Dicot WA 3 SH P 244 y y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 244 y y Allocasuarina fraseriana Fraser's Sheoak Casuarinaceae Dicot WA 1 T P 244 y y * Anagallis arvensis var. arvensis Scarlet Pimpernel Primulaceae Dicot 4 H A 244 y Anigozanthos humilis subsp. humilis Catspaw Haemodoraceae Monocot WA 4 H PAB 244 y Astartea aff. fascicularis (Gibson et al. 1994) Astartea Myrtaceae Dicot WA 3 SH P y 244 y Austrodanthonia occidentalis Western Wallaby Grass Poaceae Monocot WA 5 G P 244 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 244 y y y Banksia attenuata Candle Banksia Proteaceae Dicot WA 1 T P 244 y Banksia ilicifolia Hollyleaf Banksia Proteaceae Dicot WA 1 T P 244 y y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 244 y y Baumea articulata Jointed Twigrush Cyperaceae Monocot AUST 6 S-C P AQE y 244 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 244 y y Brachyloma preissii Globe Heath Epacridaceae Dicot WA 3 SH P 244 y y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 244 y y y * Briza minor Shivery Grass Poaceae Monocot 5 G A 244 y y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 244 y Caladenia flava subsp. -

Sistemática Y Evolución De Encyclia Hook
·>- POSGRADO EN CIENCIAS ~ BIOLÓGICAS CICY ) Centro de Investigación Científica de Yucatán, A.C. Posgrado en Ciencias Biológicas SISTEMÁTICA Y EVOLUCIÓN DE ENCYCLIA HOOK. (ORCHIDACEAE: LAELIINAE), CON ÉNFASIS EN MEGAMÉXICO 111 Tesis que presenta CARLOS LUIS LEOPARDI VERDE En opción al título de DOCTOR EN CIENCIAS (Ciencias Biológicas: Opción Recursos Naturales) Mérida, Yucatán, México Abril 2014 ( 1 CENTRO DE INVESTIGACIÓN CIENTÍFICA DE YUCATÁN, A.C. POSGRADO EN CIENCIAS BIOLÓGICAS OSCJRA )0 f CENCIAS RECONOCIMIENTO S( JIOI ÚGIC A'- CICY Por medio de la presente, hago constar que el trabajo de tesis titulado "Sistemática y evo lución de Encyclia Hook. (Orchidaceae, Laeliinae), con énfasis en Megaméxico 111" fue realizado en los laboratorios de la Unidad de Recursos Naturales del Centro de Investiga ción Científica de Yucatán , A.C. bajo la dirección de los Drs. Germán Carnevali y Gustavo A. Romero, dentro de la opción Recursos Naturales, perteneciente al Programa de Pos grado en Ciencias Biológicas de este Centro. Atentamente, Coordinador de Docencia Centro de Investigación Científica de Yucatán, A.C. Mérida, Yucatán, México; a 26 de marzo de 2014 DECLARACIÓN DE PROPIEDAD Declaro que la información contenida en la sección de Materiales y Métodos Experimentales, los Resultados y Discusión de este documento, proviene de las actividades de experimen tación realizadas durante el período que se me asignó para desarrollar mi trabajo de tesis, en las Unidades y Laboratorios del Centro de Investigación Científica de Yucatán, A.C., y que a razón de lo anterior y en contraprestación de los servicios educativos o de apoyo que me fueron brindados, dicha información, en términos de la Ley Federal del Derecho de Autor y la Ley de la Propiedad Industrial, le pertenece patrimonialmente a dicho Centro de Investigación. -

Technical Report Series No. 287 Advisory List of Environmental Weeds in Victoria
Advisory list of environmental weeds in Victoria M. White, D. Cheal, G.W. Carr, R. Adair, K. Blood and D. Meagher April 2018 Arthur Rylah Institute for Environmental Research Technical Report Series No. 287 Arthur Rylah Institute for Environmental Research Department of Environment, Land, Water and Planning PO Box 137 Heidelberg, Victoria 3084 Phone (03) 9450 8600 Website: www.ari.vic.gov.au Citation: White, M., Cheal, D., Carr, G. W., Adair, R., Blood, K. and Meagher, D. (2018). Advisory list of environmental weeds in Victoria. Arthur Rylah Institute for Environmental Research Technical Report Series No. 287. Department of Environment, Land, Water and Planning, Heidelberg, Victoria. Front cover photo: Ixia species such as I. maculata (Yellow Ixia) have escaped from gardens and are spreading in natural areas. (Photo: Kate Blood) © The State of Victoria Department of Environment, Land, Water and Planning 2018 This work is licensed under a Creative Commons Attribution 3.0 Australia licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo, the Department of Environment, Land, Water and Planning logo and the Arthur Rylah Institute logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Printed by Melbourne Polytechnic, Preston Victoria ISSN 1835-3827 (print) ISSN 1835-3835 (pdf)) ISBN 978-1-76077-000-6 (print) ISBN 978-1-76077-001-3 (pdf/online) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. -

Vegetation Patterns and Dynamics of Renosterveld at Agter-Groeneberg Conservancy, Western Cape, South Africa
Vegetation Patterns and Dynamics of Renosterveld at Agter-Groeneberg Conservancy, Western Cape, South Africa By Benjamin Alan Walton Thesis presented in partial fulfillment of the requirements for the degree of Master of Science at the Stellenbosch University Supervisor Professor Sue J Milton (Department of Conservation Ecology) Co-supervisors A le Roux (CapeNature) Professor L Mucina (Department of Botany and Zoology) April 2006 i Φ Poem “Colour awash over forelands of fertile clay” “When the winters’ cold and grim the Oxalis’s start to brim - they open up. The first feast for bees, in the shrubland short of trees not breeze. Sun’s rays soon last longer in the days: Babianas, Chlorophytums, Geissorhizas, Gladiolius’s, Hesperanthas, Lachenalias, Moraeas and Trachyandras spread their cheerful gaze. Accompanied by annual daisies and bright gladioli filling the air with strong scents of honey - monkey beetles waste no time as they perch upon delicate flowers, lest they are caught in the season’s showers. As if to suggest this is the best nature sends small midge flies to pollinate in jest, and surround mammals to tease their bloody channels. Another month has come and gone - not long now for the raaptol and Micranthus which provide nectar for brown butterflies and painted ladies. Then is the last sequence of bulbs - the fine white-filled fields of chinkerinchees. Grasses’ hour is now soaking up the sun displaying beautifully crafted silhouettes till summers end. As if heaven sent delicate geophytes are still producing their charm, when botanists avoid the midday sun. A brief lapse in displays until the autumn reds begin the seasonal cycles.” Figure a: From left to right: Moraea villosa (Ker Gawl.) Ker Gawl. -

BFS299 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No yela01 (FCT28) yela02 (FCTs07) yela03 (FCT25) Wd? Acacia cyclops Red-eyed Wattle Mimosaceae Dicot AUST 3 SH P 299 y Acacia huegelii Huegel's Wattle Mimosaceae Dicot WA 3 SH P 299 y Acacia saligna Coojong Mimosaceae Dicot WA 3 SH P 299 y * Acetosella vulgaris Sorrel Polygonaceae Dicot 4 H P 299 y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 299 y Alexgeorgea nitens Alexgeorgea Restionaceae Monocot WA 6 S-R P 299 y Allocasuarina fraseriana Fraser's Sheoak Casuarinaceae Dicot WA 1 T P 299 y Amphipogon turbinatus Amphipogon Poaceae Monocot WA 5 G P 299 y Arthropodium capillipes Summer Lily Anthericaceae Monocot WA 4 H PAB 299 y Astroloma pallidum Astroloma Epacridaceae Dicot WA 3 SH P 299 y Austrodanthonia occidentalis Western Wallaby Grass Poaceae Monocot WA 5 G P 299 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 299 y Austrostipa flavescens Tall Speargrass Poaceae Monocot AUST 5 G P 299 y * Avena fatua Wild Oat Poaceae Monocot 5 G A 299 y Banksia attenuata Candle Banksia Proteaceae Dicot WA 1 T P 299 y Banksia grandis Bull Banksia Proteaceae Dicot WA 1 T P 299 y Banksia littoralis Swamp Banksia Proteaceae Dicot WA 1 T P y 299 y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 299 y Bolboschoenus caldwellii Marsh Clubrush Cyperaceae Monocot AUST 6 S-C P AQE 299 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 299 y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 299 y y * Briza minor Shivery Grass Poaceae Monocot 5 G A 299 y * Bromus diandrus Great Brome Poaceae Monocot 5 G A 299 y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 299 y Caladenia flava subsp. -

September - November 2006
Australasian Plant Conservation BULLETIN OF THE AUSTRALIAN NETWORK FOR PLANT CONSERVATION VOLUME 15 NUMBER 2 • SEPTEMBER - NOVEMBER 2006 Role of symbiotic relationships in Australian terrestrial orchid conservation Fungi in agricultural landscapes: implications for eucalypt woodland revegetation Mycorrhizal fungi of Prasophyllum Small mammals as seed dispersers And much much more……… SPECIAL THEME: CONSERVING SYMBIOSES AUSTRALASIAN PLANT CONSERVATION This issue From the Editor: introducing ‘Conserving Symbioses’ 1 Role of symbiotic relationships in Australian terrestrial 2 orchid conservation Endophytic fungi associated with Australian orchids 7 Mycorrhizal fungi of Prasophyllum 10 ANPC Inc. Mission Statement Royal Botanic Gardens Melbourne contributes to Victorian 12 orchid conservation “To promote and improve plant conservation” Below-ground thinking: a review of the 5th International 14 Conference on Mycorrhiza Contributing to Australasian Plant Conservation Fungi in agricultural landscapes: implications for eucalypt 15 woodland revegetation Australasian Plant Conservation is a forum for information exchange for all Small mammals as seed dispersers 19 those involved in plant conservation: FloraBank lives again 21 please use it to share your work with others. Articles, information snippets, The Auckland Botanic Gardens Threatened Native Plant Garden 23 details of new publications or research, and diary dates are welcome. The deadline Regular Features for the December 2006-February 2007 issue is Friday 24 November 2006. Report from New Zealand Plant Conservation Network 25 The December-February issue will be Book Review 26 on the special theme of ‘Conserving Grasslands and Grassy Ecosystems’; Research Roundup 27 however general articles are also very Information Resources and Useful Websites 28 welcome. Please contact Tom May if you are intending to submit an article: ANPC Workshops 29 [email protected]. -

Molecular Phylogenetic Relationships Within the Subtribe Disinae (Orchidaceae) and Their Taxonomic, Phytogeographic and Evolutionary Implications
Molecular phylogenetic relationships within the subtribe Disinae (Orchidaceae) and their taxonomic, phytogeographic and evolutionary implications Benny Bytebier Dissertation presented for the degree of Doctor of Philosophy (Biochemistry) at Stellenbosch University Supervisors: Prof. Dirk U. Bellstedt, Stellenbosch University Prof. H. Peter Linder, University of Zurich Department of Biochemistry Stellenbosch University March 2007 Declaration I, the undersigned, hereby declare that the work contained in this dissertation is my own original work and that I have not previously in its entirity or in part submitted it at any university for a degree ………………………… …………………… Signature Date ii Abstract Twenty five years after the last major morphological revision, phylogenetic relationships were inferred on the basis of a new DNA dataset for the African orchid subtribe Disinae, which includes the large genus Disa and the small genus Schizodium. One nuclear gene region (ITS) and two plastid gene regions (trnLF and matK) were sequenced for 136 ingroup, representing 70% of all known Disinae species, as well as for 7 outgroup taxa. The combined data matrix contained 4094 characters and was analysed using parsimony and Bayesian inference. The generic status of Schizodium can no longer be supported, as it is deeply embedded within the genus Disa. Furthermore, the currently recognised subgenera do not reflect the phylogenetic relationships. Several of the currently recognised sections are monophyletic, others contain misplaced elements, while some are polyphyletic. These results necessitate a re-classification of the Disinae. A monotypic subtribe Disinae and a subdvision of Disa into eighteen sections is formally proposed. These sections are monophyletic, well-supported, morphologically distinguishable and are delimited to maximize the congruence with the previous classification. -

Biogeography and Ecology of Tulasnellaceae
Chapter 12 Biogeography and Ecology of Tulasnellaceae Franz Oberwinkler, Darı´o Cruz, and Juan Pablo Sua´rez 12.1 Introduction Schroter€ (1888) introduced the name Tulasnella in honour of the French physicians, botanists and mycologists Charles and Louis Rene´ Tulasne for heterobasidiomycetous fungi with unique meiosporangial morphology. The place- ment in the Heterobasidiomycetes was accepted by Rogers (1933), and later also by Donk (1972). In Talbot’s conspectus of basidiomycetes genera (Talbot 1973), the genus represented an order, the Tulasnellales, in the Holobasidiomycetidae, a view not accepted by Bandoni and Oberwinkler (1982). In molecular phylogenetic studies, Tulasnellaceae were included in Cantharellales (Hibbett and Thorn 2001), a position that was confirmed by following studies, e.g. Hibbett et al. (2007, 2014). 12.2 Systematics and Taxonomy Most tulasnelloid fungi produce basidiomata on wood, predominantly on the underside of fallen logs and twigs. Reports on these collections are mostly published in local floras, mycofloristic listings, or partial monographic treatments. F. Oberwinkler (*) Institut für Evolution und O¨ kologie, Universita¨tTübingen, Auf der Morgenstelle 1, 72076 Tübingen, Germany e-mail: [email protected] D. Cruz • J.P. Sua´rez Museum of Biological Collections, Section of Basic and Applied Biology, Department of Natural Sciences, Universidad Te´cnica Particular de Loja, San Cayetano Alto s/n C.P, 11 01 608 Loja, Ecuador © Springer International Publishing AG 2017 237 L. Tedersoo (ed.), Biogeography of Mycorrhizal Symbiosis, Ecological Studies 230, DOI 10.1007/978-3-319-56363-3_12 238 F. Oberwinkler et al. Unfortunately, the ecological relevance of Tulasnella fruiting on variously decayed wood or on bark of trees is not understood. -

Ancestral State Reconstruction of the Mycorrhizal Association for the Last Common Ancestor of Embryophyta, Given the Different Phylogenetic Constraints
Supplementary information Supplementary Figures Figure S1 | Ancestral state reconstruction of the mycorrhizal association for the last common ancestor of Embryophyta, given the different phylogenetic constraints. Pie charts show the likelihood of the ancestral states for the MRCA of Embryophyta for each phylogenetic hypothesis shown below. Letters represent mycorrhizal associations: (A) Ascomycota; (B) Basidiomycota; (G) Glomeromycotina; (M) Mucoromycotina; (-) Non-mycorrhizal. Combinations of letters represent a combination of mycorrhizal associations. Austrocedrus chilensis Chamaecyparis obtusa Sequoiadendron giganteum Prumnopitys taxifolia Prumnopitys Prumnopitys montana Prumnopitys Prumnopitys ferruginea Prumnopitys Araucaria angustifolia Araucaria Dacrycarpus dacrydioides Dacrycarpus Taxus baccata Podocarpus oleifolius Podocarpus Afrocarpus falcatus Afrocarpus Ephedra fragilis Nymphaea alba Nymphaea Gnetum gnemon Abies alba Abies balsamea Austrobaileya scandens Austrobaileya Abies nordmanniana Thalictrum minus Thalictrum Abies homolepis Caltha palustris Caltha Abies magnifica ia repens Ranunculus Abies religiosa Ranunculus montanus Ranunculus Clematis vitalba Clematis Keteleeria davidiana Anemone patens Anemone Tsuga canadensis Vitis vinifera Vitis Tsuga mertensiana Saxifraga oppositifolia Saxifraga Larix decidua Hypericum maculatum Hypericum Larix gmelinii Phyllanthus calycinus Phyllanthus Larix kaempferi Hieronyma oblonga Hieronyma Pseudotsuga menziesii Salix reinii Salix Picea abies Salix polaris Salix Picea crassifolia Salix herbacea -

The Phylogenetic Position of the Enigmatic Orchid Genus Pachites ⁎ B
Available online at www.sciencedirect.com South African Journal of Botany 74 (2008) 306–312 www.elsevier.com/locate/sajb The phylogenetic position of the enigmatic orchid genus Pachites ⁎ B. Bytebier a, , T. Van der Niet b,1, D.U. Bellstedt a, H.P. Linder c a Biochemistry Department, Stellenbosch University, Private Bag X1, Stellenbosch 7602, South Africa b School of Biological and Conservation Sciences, University of KwaZulu-Natal Pietermaritzburg, Private Bag X01, Scottsville 3209, South Africa c Institute of Systematic Botany, University of Zurich, Zollikerstrasse 107, CH-8008, Switzerland Received 10 June 2007; received in revised form 19 December 2007; accepted 9 January 2008 Abstract The orchid genus Pachites is endemic to the Cape Floristic Region and consists of two rare species that only flower during the first year after fire. Pachites has been considered closely related to Satyrium and was for that reason grouped with it in the subtribe Satyriinae. In 2005, we managed to collect material of P. bodkinii and here test the monophyly of the subtribe based on plastid DNA sequence data. We conclusively show that Satyriinae is not monophyletic in its current circumscription and that Pachites is sister to a clade comprising ((Disinae+Coryciinae s.s.)+(Satyrium+ Orchideae)). © 2008 SAAB. Published by Elsevier B.V. All rights reserved. Keywords: Cape flora; Diseae; Molecular systematics; Orchidaceae; Orchideae; Orchidoideae; Satyriinae 1. Introduction which is often subcapitate. The flowers are non-resupinate, subactinomorphic and not spurred. The two species of Pachites The orchid genus Pachites was established by John Lindley grow in dry, sandy or stony, oligotrophic soils derived from the in 1835 for Pachites appressa, a specimen of which was col- sandstones of the Cape Supergroup and are endemic to the Cape lected by William Burchell on the Langeberg near Swellendam Floristic Region (Goldblatt, 1978).