September - November 2006
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier Area, Swellendam
Biodiversity and Ecology of Critically Endangered, Rûens Silcrete Renosterveld in the Buffeljagsrivier area, Swellendam by Johannes Philippus Groenewald Thesis presented in fulfilment of the requirements for the degree of Masters in Science in Conservation Ecology in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Michael J. Samways Co-supervisor: Dr. Ruan Veldtman December 2014 Stellenbosch University http://scholar.sun.ac.za Declaration I hereby declare that the work contained in this thesis, for the degree of Master of Science in Conservation Ecology, is my own work that have not been previously published in full or in part at any other University. All work that are not my own, are acknowledge in the thesis. ___________________ Date: ____________ Groenewald J.P. Copyright © 2014 Stellenbosch University All rights reserved ii Stellenbosch University http://scholar.sun.ac.za Acknowledgements Firstly I want to thank my supervisor Prof. M. J. Samways for his guidance and patience through the years and my co-supervisor Dr. R. Veldtman for his help the past few years. This project would not have been possible without the help of Prof. H. Geertsema, who helped me with the identification of the Lepidoptera and other insect caught in the study area. Also want to thank Dr. K. Oberlander for the help with the identification of the Oxalis species found in the study area and Flora Cameron from CREW with the identification of some of the special plants growing in the area. I further express my gratitude to Dr. Odette Curtis from the Overberg Renosterveld Project, who helped with the identification of the rare species found in the study area as well as information about grazing and burning of Renosterveld. -

An Asian Orchid, Eulophia Graminea (Orchidaceae: Cymbidieae), Naturalizes in Florida
LANKESTERIANA 8(1): 5-14. 2008. AN ASIAN ORCHID, EULOPHIA GRAMINEA (ORCHIDACEAE: CYMBIDIEAE), NATURALIZES IN FLORIDA ROBE R T W. PEMBE R TON 1,3, TIMOTHY M. COLLINS 2 & SUZANNE KO P TU R 2 1Fairchild Tropical Botanic Garden, 2121 SW 28th Terrace Ft. Lauderdale, Florida 33312 2Department of Biological Sciences, Florida International University, Miami, FL 33199 3Author for correspondence: [email protected] ABST R A C T . Eulophia graminea, a terrestrial orchid native to Asia, has naturalized in southern Florida. Orchids naturalize less often than other flowering plants or ferns, butE. graminea has also recently become naturalized in Australia. Plants were found growing in five neighborhoods in Miami-Dade County, spanning 35 km from the most northern to the most southern site, and growing only in woodchip mulch at four of the sites. Plants at four sites bore flowers, and fruit were observed at two sites. Hand pollination treatments determined that the flowers are self compatible but fewer fruit were set in selfed flowers (4/10) than in out-crossed flowers (10/10). No fruit set occurred in plants isolated from pollinators, indicating that E. graminea is not autogamous. Pollinia removal was not detected at one site, but was 24.3 % at the other site evaluated for reproductive success. A total of 26 and 92 fruit were found at these two sites, where an average of 6.5 and 3.4 fruit were produced per plant. These fruits ripened and dehisced rapidly; some dehiscing while their inflorescences still bore open flowers. Fruit set averaged 9.2 and 4.5 % at the two sites. -

ACT, Australian Capital Territory
Biodiversity Summary for NRM Regions Species List What is the summary for and where does it come from? This list has been produced by the Department of Sustainability, Environment, Water, Population and Communities (SEWPC) for the Natural Resource Management Spatial Information System. The list was produced using the AustralianAustralian Natural Natural Heritage Heritage Assessment Assessment Tool Tool (ANHAT), which analyses data from a range of plant and animal surveys and collections from across Australia to automatically generate a report for each NRM region. Data sources (Appendix 2) include national and state herbaria, museums, state governments, CSIRO, Birds Australia and a range of surveys conducted by or for DEWHA. For each family of plant and animal covered by ANHAT (Appendix 1), this document gives the number of species in the country and how many of them are found in the region. It also identifies species listed as Vulnerable, Critically Endangered, Endangered or Conservation Dependent under the EPBC Act. A biodiversity summary for this region is also available. For more information please see: www.environment.gov.au/heritage/anhat/index.html Limitations • ANHAT currently contains information on the distribution of over 30,000 Australian taxa. This includes all mammals, birds, reptiles, frogs and fish, 137 families of vascular plants (over 15,000 species) and a range of invertebrate groups. Groups notnot yet yet covered covered in inANHAT ANHAT are notnot included included in in the the list. list. • The data used come from authoritative sources, but they are not perfect. All species names have been confirmed as valid species names, but it is not possible to confirm all species locations. -
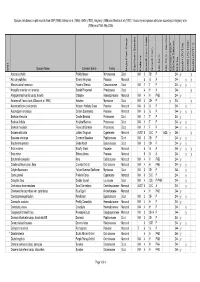
BFS244 Site Species List
Species lists based on plot records from DEP (1996), Gibson et al. (1994), Griffin (1993), Keighery (1996) and Weston et al. (1992). Taxonomy and species attributes according to Keighery et al. (2006) as of 16th May 2005. Species Name Common Name Family Major Plant Group Significant Species Endemic Growth Form Code Growth Form Life Form Life Form - aquatics Common SSCP Wetland Species BFS No beel01 (FCT28) beel02 (FCT23a) beel03 (FCT11) Wd? Acacia pulchella Prickly Moses Mimosaceae Dicot WA 3 SH P 244 y y * Aira caryophyllea Silvery Hairgrass Poaceae Monocot 5 G A 244 y y Allocasuarina fraseriana Fraser's Sheoak Casuarinaceae Dicot WA 1 T P 244 y y * Anagallis arvensis var. arvensis Scarlet Pimpernel Primulaceae Dicot 4 H A 244 y Anigozanthos humilis subsp. humilis Catspaw Haemodoraceae Monocot WA 4 H PAB 244 y Astartea aff. fascicularis (Gibson et al. 1994) Astartea Myrtaceae Dicot WA 3 SH P y 244 y Austrodanthonia occidentalis Western Wallaby Grass Poaceae Monocot WA 5 G P 244 y Austrostipa compressa Golden Speargrass Poaceae Monocot WA 5 G P 244 y y y Banksia attenuata Candle Banksia Proteaceae Dicot WA 1 T P 244 y Banksia ilicifolia Hollyleaf Banksia Proteaceae Dicot WA 1 T P 244 y y Banksia menziesii Firewood Banksia Proteaceae Dicot WA 1 T P 244 y y Baumea articulata Jointed Twigrush Cyperaceae Monocot AUST 6 S-C P AQE y 244 y Bossiaea eriocarpa Common Bossiaea Papilionaceae Dicot WA 3 SH P 244 y y Brachyloma preissii Globe Heath Epacridaceae Dicot WA 3 SH P 244 y y * Briza maxima Blowfly Grass Poaceae Monocot 5 G A 244 y y y * Briza minor Shivery Grass Poaceae Monocot 5 G A 244 y y Burchardia congesta Kara Colchicaceae Monocot WA 4 H PAB 244 y Caladenia flava subsp. -

Romano's Investment Holdings Flora and Vegetation Assessment Lot 54
Romano’s Investment Holdings Flora and Vegetation Assessment Lot 54 and Lot 9001 Holden Close, Bertram January 2014 Romano’s Investment Holdings Flora and Vegetation Assessment Lot 54 and Lot 9001 Holden Close, Bertram January 2014 Report Reference No. ENAUPERT04366A_01_v2 Disclaimer This document is published in accordance with and subject to an agreement between Coffey and the client for whom it has been prepared, Romano’s Investment Holdings (‘Client’), and is restricted to those issues that have been raised by the client in its engagement of Coffey and prepared using the standard of skill and care ordinarily exercised by environmental scientists in the preparation of such documents. Any person or organisation that relies on or uses the document for purposes or reasons other than those agreed by Coffey and the Client without first obtaining the prior written consent of Coffey, does so entirely at their own risk and Coffey denies all liability in tort, contract or otherwise for any loss, damage or injury of any kind whatsoever (whether in negligence or otherwise) that may be suffered as a consequence of relying on this document for any purpose other than that agreed with the Client. © Coffey Environments Australia Pty Ltd ABN 65140765902. 21 January 2014. Suite 2, 53 Burswood Road Burswood WA 6100 Australia PO Box 4223 Victoria Park WA 6979 Australia t +61 8 9355 7100 f +61 8 9355 7111 coffey.com Library Reference No.: EP2013/211 Report Reference No.: ENAUPERT04366AA_01_V2 Project Director M. Scheltema Project Manager M. Johnston Record of Distribution Report Status: No. of Format Distributed to Date Authorised by copies V1 (draft) 1 PDF RPS 10/12/2013 M. -

Sistemática Y Evolución De Encyclia Hook
·>- POSGRADO EN CIENCIAS ~ BIOLÓGICAS CICY ) Centro de Investigación Científica de Yucatán, A.C. Posgrado en Ciencias Biológicas SISTEMÁTICA Y EVOLUCIÓN DE ENCYCLIA HOOK. (ORCHIDACEAE: LAELIINAE), CON ÉNFASIS EN MEGAMÉXICO 111 Tesis que presenta CARLOS LUIS LEOPARDI VERDE En opción al título de DOCTOR EN CIENCIAS (Ciencias Biológicas: Opción Recursos Naturales) Mérida, Yucatán, México Abril 2014 ( 1 CENTRO DE INVESTIGACIÓN CIENTÍFICA DE YUCATÁN, A.C. POSGRADO EN CIENCIAS BIOLÓGICAS OSCJRA )0 f CENCIAS RECONOCIMIENTO S( JIOI ÚGIC A'- CICY Por medio de la presente, hago constar que el trabajo de tesis titulado "Sistemática y evo lución de Encyclia Hook. (Orchidaceae, Laeliinae), con énfasis en Megaméxico 111" fue realizado en los laboratorios de la Unidad de Recursos Naturales del Centro de Investiga ción Científica de Yucatán , A.C. bajo la dirección de los Drs. Germán Carnevali y Gustavo A. Romero, dentro de la opción Recursos Naturales, perteneciente al Programa de Pos grado en Ciencias Biológicas de este Centro. Atentamente, Coordinador de Docencia Centro de Investigación Científica de Yucatán, A.C. Mérida, Yucatán, México; a 26 de marzo de 2014 DECLARACIÓN DE PROPIEDAD Declaro que la información contenida en la sección de Materiales y Métodos Experimentales, los Resultados y Discusión de este documento, proviene de las actividades de experimen tación realizadas durante el período que se me asignó para desarrollar mi trabajo de tesis, en las Unidades y Laboratorios del Centro de Investigación Científica de Yucatán, A.C., y que a razón de lo anterior y en contraprestación de los servicios educativos o de apoyo que me fueron brindados, dicha información, en términos de la Ley Federal del Derecho de Autor y la Ley de la Propiedad Industrial, le pertenece patrimonialmente a dicho Centro de Investigación. -

Branch Circus Flora and Fauna Survey PDF Document
FLORA AND VEGETATION SURVEY Branch Circus and Hammond Road, Success Prepared by: Prepared for: RPS MUNTOC PTY LTD AND 290 Churchill Avenue, SUBIACO WA 6008 SILVERSTONE ASSET PTY LTD PO Box 465, SUBIACO WA 6904 C/O Koltasz Smith T: 618 9382 4744 PO Box 127 F: 618 9382 1177 E: [email protected] BURSWOOD WA 6100 W: www.rpsgroup.com.au Report No: L07263 Version/Date: Rev 0, June 2008 RPS Environment Pty Ltd (ABN 45 108 680 977) Document Set ID: 5546761 Version: 1, Version Date: 31/01/2017 Flora and Vegetation Survey Branch Circus and Hammond Road, Success Document Status Review Format RPS Release Issue Version Purpose of Document Orig Review Date Review Approval Date Draft A Draft For Internal Review KelMcC VanYeo 30.04.08 Draft B Draft For Client Review VanYeo KarGod 14.05.08 SN 30.05.08 Rev 0 Final for Issue VanYeo 10.06.08 DC 12.06.08 B. Hollyock 13.06.08 Disclaimer This document is and shall remain the property of RPS. The document may only be used for the purposes for which it was commissioned and in accordance with the Terms of Engagement for the commission. Unauthorised copying or use of this document in any form whatsoever is prohibited. L07263, Rev 0, June 2008 DOCUMENT STATUS / DISCLAIMER Document Set ID: 5546761 Version: 1, Version Date: 31/01/2017 Flora and Vegetation Survey Branch Circus and Hammond Road, Success EXECUTIVE SUMMARY Flora A total of 229 taxa were recorded from the survey area, of which 155 or 68% were native. -

Australian Orchidaceae: Genera and Species (12/1/2004)
AUSTRALIAN ORCHID NAME INDEX (21/1/2008) by Mark A. Clements Centre for Plant Biodiversity Research/Australian National Herbarium GPO Box 1600 Canberra ACT 2601 Australia Corresponding author: [email protected] INTRODUCTION The Australian Orchid Name Index (AONI) provides the currently accepted scientific names, together with their synonyms, of all Australian orchids including those in external territories. The appropriate scientific name for each orchid taxon is based on data published in the scientific or historical literature, and/or from study of the relevant type specimens or illustrations and study of taxa as herbarium specimens, in the field or in the living state. Structure of the index: Genera and species are listed alphabetically. Accepted names for taxa are in bold, followed by the author(s), place and date of publication, details of the type(s), including where it is held and assessment of its status. The institution(s) where type specimen(s) are housed are recorded using the international codes for Herbaria (Appendix 1) as listed in Holmgren et al’s Index Herbariorum (1981) continuously updated, see [http://sciweb.nybg.org/science2/IndexHerbariorum.asp]. Citation of authors follows Brummit & Powell (1992) Authors of Plant Names; for book abbreviations, the standard is Taxonomic Literature, 2nd edn. (Stafleu & Cowan 1976-88; supplements, 1992-2000); and periodicals are abbreviated according to B-P- H/S (Bridson, 1992) [http://www.ipni.org/index.html]. Synonyms are provided with relevant information on place of publication and details of the type(s). They are indented and listed in chronological order under the accepted taxon name. Synonyms are also cross-referenced under genus. -

Fungal Endophytes in Australian Myco-Heterotrophic Orchids
Fungal Endophytes in Australian Myco-heterotrophic Orchids J.D.W. Dearnaley Centre for Systems Biology, The University of Southern Queensland, Toowoomba, Australia Summary The fungal endophytes of Australian myco-heterotrophic orchids are largely unknown. In this investigation we identified the fungal endophytes of three species of Australian myco-heterotrophic orchid: the terrestrial species Dipodium variegatum and Dipodium hamiltonianum and the vine-like Erythrorchis cassythoides. Similar to studies of myco-heterotrophic orchids in Nth America and Europe the fungal endophytes of Dipodium spp. are members of the Russulaceae. E. cassythoides contained both ectomycorrhizal and saprotrophic fungi suggesting that it can derive its carbon via fungi from both dead and living plant material. Introduction All orchids rely on fungi for seed germination and early growth. A number of orchid species are non-photosynthetic and are termed myco-heterotrophic because of their complete dependence on fungi throughout their life cycle. Molecular identification studies of fungal endophytes of myco-heterotrophic orchids have shown that these plants are colonized by higher basidiomycetes such as members of the Russulaceae and Thelophoraceae (Taylor and Bruns 1997, 1999, Girlanda et al. 2006) as well as members of the heterobasidomycete family Sebacinaceae (Selosse et al. 2002, Taylor et al. 2003). As these fungal groups contain ectomycorrhizal species it appears that myco-heterotrophic orchids are indirectly parasitic on the tree host partner of ectomycorrhizal partnerships. Evidence supporting this comes from McKendrick et al. (2000) who have shown that radiolabelled carbon can flow from tree to myco-hetero- trophic orchid via a fungal conduit, additionally, many myco-heterotrophic ©2006 by MEDIMOND S.r.l. -

WHAT DRIVES ORCHIDS.Pmd
J. Orchid Soc. India, 29: 23-30, 2015 ISSN 0971-5371 WHAT DRIVES ORCHIDS TOWARD MYCO-HETEROTROPHY? Julie Thakur, Akankankanksha Madan, Mayyyank D Dwivedi, and Prem L Uniyalalal Department of Botany, University of Delhi, Delhi-110 007, India Abstract Myco-heterotrophy describes the capability of orchids to obtain carbon from fungi. Depending upon varied durations of association with fungi, orchids have been categorized as those that require fungi during germination only as compared to those that are associated with fungi throughout the life span. It has always been the subject of interest to all the scientists to evaluate the mode of nutrition between this mutualistic association for which various modern approaches have been used. There are factors which led to evolution of autotrophy to initial or partial mycoheterotrophy and subsequently, to fully mycoheterotrophy. The present paper presents the overview of mycoheterotrophy, habitat, morphology, reproduction strategies, techniques, threats and conservation of mycoheterotrophy, and major opportunities for the future research. Introduction is divided into five sub-families: Apostasioideae, Vanilloideae, Cypripedioideae, Orchidoideae and MYCO-HETEROTROPHY IS a potential of a plant to Epidendroideae, of which Epidendroideae is the largest procure carbon from fungi. Leake (1994) coined the subfamily comprising of ca. 18,000 spp. and 650 term mycoheterotrophy (as myco-heterotrophy). To genera (Chase et al., 2015). As far as is known, all understand mycoheterotrophy, a basic understanding orchids depend on a myco-heterotrophic interaction about the mycorrhizal symbiosis is a pre-requisite. The with a symbiotic fungus for a part of their life cycle, term mycorrhiza is derived from the Greek words, especially for germination (Leake, 1994). -

Research Article Ultrastructural Studies and Molecular Characterization of Root-Associated Fungi of Crepidium Acuminatum (D
1 Research article Ultrastructural studies and molecular characterization of root-associated fungi of Crepidium acuminatum (D. Don) Szlach.: A threatened and medicinally important taxon JULIE THAKUR, MAYANK D. DWIVEDI# and PREM L. UNIYAL Department of Botany, University of Delhi, Delhi-110007 # For correspondence. E-mail: [email protected] Abstract Crepidium acuminatum (Orchidaceae) is a threatened medicinal orchid that grows under shady and moist forest floor where light remains for a very short period of time. Mycorrhizal association is known to be essential for seed germination and seedling establishment in majority of orchids. Identification of fungi that form mycorrhizae with orchids is of crucial importance for orchid conservation. We used both morphological as well as molecular approaches to study this plant-fungal interaction. Scanning electron microscopy showed that fungi grow and proliferate in the middle layers of cortex. Also, spiral root hairs were found along with roots hairs, which is an unusual observation. Spiral root hairs provide more surface area for fluid absorption and entrance of colonisers. Furthermore, total root genomic DNA was isolated and fungal ITS regions were PCR-amplified using specific primer combinations ITS1F/ITS4 and ITS1/ITS4tul. ITS sequences were obtained and analysed to know the closest sequence matches in the GenBank using BLASTn hosted by NLM-NCBI. Subject sequences were identified to be belonging to three main genera viz., Tulasnella, Aspergillus and Penicillium. Results indicate that mycorrhizal association is necessary for the growth and development of this plant. In addition, this symbiosis influences the distribution and rarity of this medicinally valuable taxon. Specific fungal partners may lead to enhanced seed germination rate and increased efficiency of nutrient exchange between both the partners. -

Technical Report Series No. 287 Advisory List of Environmental Weeds in Victoria
Advisory list of environmental weeds in Victoria M. White, D. Cheal, G.W. Carr, R. Adair, K. Blood and D. Meagher April 2018 Arthur Rylah Institute for Environmental Research Technical Report Series No. 287 Arthur Rylah Institute for Environmental Research Department of Environment, Land, Water and Planning PO Box 137 Heidelberg, Victoria 3084 Phone (03) 9450 8600 Website: www.ari.vic.gov.au Citation: White, M., Cheal, D., Carr, G. W., Adair, R., Blood, K. and Meagher, D. (2018). Advisory list of environmental weeds in Victoria. Arthur Rylah Institute for Environmental Research Technical Report Series No. 287. Department of Environment, Land, Water and Planning, Heidelberg, Victoria. Front cover photo: Ixia species such as I. maculata (Yellow Ixia) have escaped from gardens and are spreading in natural areas. (Photo: Kate Blood) © The State of Victoria Department of Environment, Land, Water and Planning 2018 This work is licensed under a Creative Commons Attribution 3.0 Australia licence. You are free to re-use the work under that licence, on the condition that you credit the State of Victoria as author. The licence does not apply to any images, photographs or branding, including the Victorian Coat of Arms, the Victorian Government logo, the Department of Environment, Land, Water and Planning logo and the Arthur Rylah Institute logo. To view a copy of this licence, visit http://creativecommons.org/licenses/by/3.0/au/deed.en Printed by Melbourne Polytechnic, Preston Victoria ISSN 1835-3827 (print) ISSN 1835-3835 (pdf)) ISBN 978-1-76077-000-6 (print) ISBN 978-1-76077-001-3 (pdf/online) Disclaimer This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication.