Final Korrekturen 3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytokine Nomenclature
RayBiotech, Inc. The protein array pioneer company Cytokine Nomenclature Cytokine Name Official Full Name Genbank Related Names Symbol 4-1BB TNFRSF Tumor necrosis factor NP_001552 CD137, ILA, 4-1BB ligand receptor 9 receptor superfamily .2. member 9 6Ckine CCL21 6-Cysteine Chemokine NM_002989 Small-inducible cytokine A21, Beta chemokine exodus-2, Secondary lymphoid-tissue chemokine, SLC, SCYA21 ACE ACE Angiotensin-converting NP_000780 CD143, DCP, DCP1 enzyme .1. NP_690043 .1. ACE-2 ACE2 Angiotensin-converting NP_068576 ACE-related carboxypeptidase, enzyme 2 .1 Angiotensin-converting enzyme homolog ACTH ACTH Adrenocorticotropic NP_000930 POMC, Pro-opiomelanocortin, hormone .1. Corticotropin-lipotropin, NPP, NP_001030 Melanotropin gamma, Gamma- 333.1 MSH, Potential peptide, Corticotropin, Melanotropin alpha, Alpha-MSH, Corticotropin-like intermediary peptide, CLIP, Lipotropin beta, Beta-LPH, Lipotropin gamma, Gamma-LPH, Melanotropin beta, Beta-MSH, Beta-endorphin, Met-enkephalin ACTHR ACTHR Adrenocorticotropic NP_000520 Melanocortin receptor 2, MC2-R hormone receptor .1 Activin A INHBA Activin A NM_002192 Activin beta-A chain, Erythroid differentiation protein, EDF, INHBA Activin B INHBB Activin B NM_002193 Inhibin beta B chain, Activin beta-B chain Activin C INHBC Activin C NM005538 Inhibin, beta C Activin RIA ACVR1 Activin receptor type-1 NM_001105 Activin receptor type I, ACTR-I, Serine/threonine-protein kinase receptor R1, SKR1, Activin receptor-like kinase 2, ALK-2, TGF-B superfamily receptor type I, TSR-I, ACVRLK2 Activin RIB ACVR1B -

Oncostatin M Exhibit Elevated Responsiveness to IL-31 Receptor
IL-31 Receptor (IL-31RA) Knockout Mice Exhibit Elevated Responsiveness to Oncostatin M This information is current as Janine Bilsborough, Sherri Mudri, Eric Chadwick, Brandon of September 28, 2021. Harder and Stacey R. Dillon J Immunol 2010; 185:6023-6030; Prepublished online 18 October 2010; doi: 10.4049/jimmunol.0902769 http://www.jimmunol.org/content/185/10/6023 Downloaded from References This article cites 29 articles, 6 of which you can access for free at: http://www.jimmunol.org/content/185/10/6023.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication by guest on September 28, 2021 *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology IL-31 Receptor (IL-31RA) Knockout Mice Exhibit Elevated Responsiveness to Oncostatin M Janine Bilsborough,1 Sherri Mudri,1 Eric Chadwick,2 Brandon Harder,3 and Stacey R. Dillon IL-31 signals through the heterodimeric receptor IL-31RA and oncostatin M receptor (OSMR), and has been linked with the development of atopic dermatitis, a Th2 cytokine-associated disease in humans. -

System, Method and Software for Calculation of a Cannabis Drug Efficiency Index for the Reduction of Inflammation
International Journal of Molecular Sciences Article System, Method and Software for Calculation of a Cannabis Drug Efficiency Index for the Reduction of Inflammation Nicolas Borisov 1,† , Yaroslav Ilnytskyy 2,3,†, Boseon Byeon 2,3,4,†, Olga Kovalchuk 2,3 and Igor Kovalchuk 2,3,* 1 Moscow Institute of Physics and Technology, 9 Institutsky lane, Dolgoprudny, Moscow Region 141701, Russia; [email protected] 2 Department of Biological Sciences, University of Lethbridge, Lethbridge, AB T1K 3M4, Canada; [email protected] (Y.I.); [email protected] (B.B.); [email protected] (O.K.) 3 Pathway Rx., 16 Sandstone Rd. S., Lethbridge, AB T1K 7X8, Canada 4 Biomedical and Health Informatics, Computer Science Department, State University of New York, 2 S Clinton St, Syracuse, NY 13202, USA * Correspondence: [email protected] † First three authors contributed equally to this research. Abstract: There are many varieties of Cannabis sativa that differ from each other by composition of cannabinoids, terpenes and other molecules. The medicinal properties of these cultivars are often very different, with some being more efficient than others. This report describes the development of a method and software for the analysis of the efficiency of various cannabis extracts to detect the anti-inflammatory properties of the various cannabis extracts. The method uses high-throughput gene expression profiling data but can potentially use other omics data as well. According to the signaling pathway topology, the gene expression profiles are convoluted into the signaling pathway activities using a signaling pathway impact analysis (SPIA) method. The method was tested by inducing inflammation in human 3D epithelial tissues, including intestine, oral and skin, and then exposing these tissues to various extracts and then performing transcriptome analysis. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -

IL-31 Uncouples Skin Inflammation from Itch Sensation in Allergic Dermatitis
UCSF UC San Francisco Previously Published Works Title IL-31 uncouples skin inflammation from itch sensation in allergic dermatitis Permalink https://escholarship.org/uc/item/9sh4x406 Authors Fassett, Marlys S Braz, Joao M Castellanos, Carlos A et al. Publication Date 2021-05-13 DOI 10.1101/2021.05.12.443916 Peer reviewed eScholarship.org Powered by the California Digital Library University of California bioRxiv preprint doi: https://doi.org/10.1101/2021.05.12.443916; this version posted May 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Title: IL-31 uncouples skin inflammation from itch sensation in allergic dermatitis Authors: Marlys S. Fassett,1,2,3 Joao M. Braz,4 Carlos A. Castellanos,2,3 Andrew W. Schroeder,3 Mahsa Sadeghi,4 Darryl J. Mar,2 Connie J. Zhou,2 Jeoung-Sook Shin,2,3 Allan I. Basbaum,4 and K. Mark Ansel2,3 Affiliations: 1Department of Dermatology, University of California, San Francisco; San Francisco, USA 2Department of Microbiology and Immunology, University of California, San Francisco; San Francisco, USA. 3Sandler Asthma Basic Research Center (SABRe); San Francisco, USA. 4Department of Anatomy, University of California San Francisco; San Francisco, USA. *Corresponding author. Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.05.12.443916; this version posted May 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. ABSTRACT Despite a robust literature associating IL-31 with pruritic inflammatory skin diseases, its influence on cutaneous inflammation and on the interplay between inflammatory and neurosensory pathways remain unmapped. -
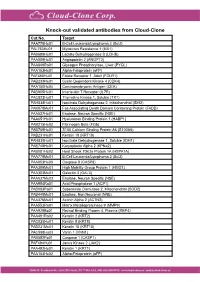
Knock-Out Validated Antibodies from Cloud-Clone Cat.No
Knock-out validated antibodies from Cloud-Clone Cat.No. Target PAA778Hu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAL763Hu01 Myxovirus Resistance 1 (MX1) PAB698Hu01 Lactate Dehydrogenase B (LDHB) PAA009Hu01 Angiopoietin 2 (ANGPT2) PAA849Ra01 Glycogen Phosphorylase, Liver (PYGL) PAA153Hu01 Alpha-Fetoprotein (aFP) PAF460Hu01 Folate Receptor 1, Adult (FOLR1) PAB233Hu01 Cyclin Dependent Kinase 4 (CDK4) PAA150Hu04 Carcinoembryonic Antigen (CEA) PAB905Hu01 Interleukin 7 Receptor (IL7R) PAC823Hu01 Thymidine Kinase 1, Soluble (TK1) PAH838Hu01 Isocitrate Dehydrogenase 2, mitochondrial (IDH2) PAK078Mu01 Fas Associating Death Domain Containing Protein (FADD) PAA537Hu01 Enolase, Neuron Specific (NSE) PAA651Hu01 Hyaluronan Binding Protein 1 (HABP1) PAB215Hu02 Fibrinogen Beta (FGb) PAB769Hu01 S100 Calcium Binding Protein A6 (S100A6) PAB231Hu01 Keratin 18 (KRT18) PAH839Hu01 Isocitrate Dehydrogenase 1, Soluble (IDH1) PAE748Hu01 Karyopherin Alpha 2 (KPNa2) PAB081Hu02 Heat Shock 70kDa Protein 1A (HSPA1A) PAA778Mu01 B-Cell Leukemia/Lymphoma 2 (Bcl2) PAA853Hu03 Caspase 8 (CASP8) PAA399Mu01 High Mobility Group Protein 1 (HMG1) PAA303Mu01 Galectin 3 (GAL3) PAA537Mu02 Enolase, Neuron Specific (NSE) PAA994Ra01 Acid Phosphatase 1 (ACP1) PAB083Ra01 Superoxide Dismutase 2, Mitochondrial (SOD2) PAB449Mu01 Enolase, Non Neuronal (NNE) PAA376Mu01 Actinin Alpha 2 (ACTN2) PAA553Ra01 Matrix Metalloproteinase 9 (MMP9) PAA929Bo01 Retinol Binding Protein 4, Plasma (RBP4) PAA491Ra02 Keratin 2 (KRT2) PAC025Hu01 Keratin 8 (KRT8) PAB231Mu01 Keratin 18 (KRT18) PAC598Hu03 Vanin 1 (VNN1) -

Evolutionary Divergence and Functions of the Human Interleukin (IL) Gene Family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W
UPDATE ON GENE COMPLETIONS AND ANNOTATIONS Evolutionary divergence and functions of the human interleukin (IL) gene family Chad Brocker,1 David Thompson,2 Akiko Matsumoto,1 Daniel W. Nebert3* and Vasilis Vasiliou1 1Molecular Toxicology and Environmental Health Sciences Program, Department of Pharmaceutical Sciences, University of Colorado Denver, Aurora, CO 80045, USA 2Department of Clinical Pharmacy, University of Colorado Denver, Aurora, CO 80045, USA 3Department of Environmental Health and Center for Environmental Genetics (CEG), University of Cincinnati Medical Center, Cincinnati, OH 45267–0056, USA *Correspondence to: Tel: þ1 513 821 4664; Fax: þ1 513 558 0925; E-mail: [email protected]; [email protected] Date received (in revised form): 22nd September 2010 Abstract Cytokines play a very important role in nearly all aspects of inflammation and immunity. The term ‘interleukin’ (IL) has been used to describe a group of cytokines with complex immunomodulatory functions — including cell proliferation, maturation, migration and adhesion. These cytokines also play an important role in immune cell differentiation and activation. Determining the exact function of a particular cytokine is complicated by the influence of the producing cell type, the responding cell type and the phase of the immune response. ILs can also have pro- and anti-inflammatory effects, further complicating their characterisation. These molecules are under constant pressure to evolve due to continual competition between the host’s immune system and infecting organisms; as such, ILs have undergone significant evolution. This has resulted in little amino acid conservation between orthologous proteins, which further complicates the gene family organisation. Within the literature there are a number of overlapping nomenclature and classification systems derived from biological function, receptor-binding properties and originating cell type. -

Np63 Regulates IL-33 and IL-31 Signaling in Atopic Dermatitis
Cell Death and Differentiation (2016) 23, 1073–1085 OPEN & 2016 Macmillan Publishers Limited All rights reserved 1350-9047/16 www.nature.com/cdd ΔNp63 regulates IL-33 and IL-31 signaling in atopic dermatitis JM Rizzo1,2, A Oyelakin3, S Min3, K Smalley1, J Bard1, W Luo1,7, J Nyquist4, E Guttman-Yassky5, T Yoshida6, A De Benedetto6, LA Beck6, S Sinha*,1 and R-A Romano*,3 Atopic dermatitis (AD) is the most common inflammatory skin disease with no well-delineated cause or effective cure. Here we show that the p53 family member p63, specifically the ΔNp63, isoform has a key role in driving keratinocyte activation in AD. We find that overexpression of ΔNp63 in transgenic mouse epidermis results in a severe skin phenotype that shares many of the key clinical, histological and molecular features associated with human AD. This includes pruritus, epidermal hyperplasia, aberrant keratinocyte differentiation, enhanced expression of selected cytokines and chemokines and the infiltration of large numbers of inflammatory cells including type 2 T-helper cells – features that are highly representative of AD dermatopathology. We further demonstrate several of these mediators to be direct transcriptional targets of ΔNp63 in keratinocytes. Of particular significance are two p63 target genes, IL-31 and IL-33, both of which are key players in the signaling pathways implicated in AD. Importantly, we find these observations to be in good agreement with elevated levels of ΔNp63 in skin lesions of human patients with AD. Our studies reveal an important role for ΔNp63 in the pathogenesis of AD and offer new insights into its etiology and possible therapeutic targets. -

Differentially Methylated Genes
10/30/2013 Disclosures Key Rheumatoid Arthritis-Associated Pathogenic Pathways Revealed by Integrative Analysis of RA Omics Datasets Consultant: IGNYTA Funding: Rheumatology Research Foundation By John W. Whitaker, Wei Wang and Gary S. Firestein DNA methylation and gene regulation The RA methylation signature in FLS DNA methylation – DNMT1 (maintaining methylation) OA – DNMT3a, 3b (de novo methylation) RA % of CpG methylation: 0% 100% Nakano et al. 2013 ARD AA06 AANAT AARS ABCA6 ABCC12 ABCG1 ABHD8 ABL2 ABR ABRA ACACA ACAN ACAP3 ACCSL ACN9 ACOT7 ACOX2 ACP5 ACP6 ACPP ACSL1 ACSL3 ACSM5 ACVRL1 ADAM10 ADAM32 ADAM33 ADAMTS12 ADAMTS15 ADAMTS19 ADAMTS4 ADAT3 ADCK4 ADCK5 ADCY2 ADCY3 ADCY6 ADORA1 ADPGK ADPRHL1 ADTRP AFAP1 AFAP1L2 AFF3 AFG3L1P AGAP11 AGER AGTR1 AGXT AIF1L AIM2 AIRE AJUBA AK4 AKAP12 AKAP2 AKR1C2 AKR1E2 AKT2 ALAS1 ALDH1L1-AS1 ALDH3A1 ALDH3B1 ALDH8A1 ALDOB ALDOC ALOX12 ALPK3 ALS2CL ALX4 AMBRA1 AMPD2 AMPD3 ANGPT1 ANGPT2 ANGPTL5 ANGPTL6 ANK1 ANKMY2 ANKRD29 ANKRD37 ANKRD53 ANO3 ANO6 ANO7 ANP32C ANXA6 ANXA8L2 AP1G1 AP2A2 AP2M1 AP5B1 APBA2 APC APCDD1 APOBEC3B APOBEC3G APOC1 APOH APOL6 APOLD1 APOM AQP1 AQP10 AQP6 AQP9 ARAP1 ARHGAP24 ARHGAP42 ARHGEF19 ARHGEF25 ARHGEF3 ARHGEF37 ARHGEF7 ARL4C ARL6IP 5 ARL8B ARMC3 ARNTL2 ARPP21 ARRB1 ARSI ASAH2B ASB10 ASB2 ASCL2 ASIC4 ASPH ATF3 ATF7 ATL1 ATL3 ATP10A ATP1A1 ATP1A4 ATP2C1 ATP5A1 ATP5EP2 ATP5L2 ATP6V0CP3 ATP6V1C1 ATP6V1E2 ATXN7L1 ATXN7L2 AVPI1 AXIN2 B3GNT7 B3GNT8 B3GNTL1 BACH1 BAG3 Differential methylated genes in RA FLS BAIAP2L2 BANP BATF BATF2 BBS2 BCAS4 BCAT1 BCL7C BDKRB2 BEGAIN BEST1 BEST3 -

7/23/2021 Human IL-31 Protein, His Tag (MALS Verified) Catalog # IL1-H5247
Human IL-31 Protein, His Tag (MALS verified) Catalog # IL1-H5247 Synonym Formulation IL-31,IL31,Interleukin-31 Lyophilized from 0.22 μm filtered solution in PBS, pH7.4. Normally trehalose is Source added as protectant before lyophilization. Human IL-31, His Tag (IL1-H5247) is expressed from human 293 cells Contact us for customized product form or formulation. (HEK293). It contains AA Ser 24 - Thr 164 (Accession # Q6EBC2-1). Reconstitution Predicted N-terminus: His Molecular Characterization Please see Certificate of Analysis for specific instructions. For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA. Storage This protein carries a polyhistidine tag at the N-terminus. For long term storage, the product should be stored at lyophilized state at -20°C The protein has a calculated MW of 17.7 kDa. The protein migrates as 23-33 or lower. kDa under reducing (R) condition (SDS-PAGE) due to glycosylation. Please avoid repeated freeze-thaw cycles. Endotoxin This product is stable after storage at: Less than 1.0 EU per μg by the LAL method. -20°C to -70°C for 12 months in lyophilized state; -70°C for 3 months under sterile conditions after reconstitution. Purity >90% as determined by SDS-PAGE. >90% as determined by SEC-MALS. SDS-PAGE SEC-MALS Human IL-31, His Tag on SDS-PAGE under reducing (R) condition. The gel The purity of Human IL-31, His Tag (Cat. No. IL1-H5247) was more than 90% was stained overnight with Coomassie Blue. The purity of the protein is greater and the molecular weight of this protein is around 15-25 kDa verified by SEC- than 90%. -

Human Colon Cancer Primer Library
Human Colon Cancer Primer Library Catalog No: HCCR-1 Supplier: RealTimePrimers Lot No: XXXXX Supplied as: solid Stability: store at -20°C Description Contains 88 primer sets directed against cytokine and chemokine receptor genes and 8 housekeeping gene primer sets. Provided in a 96-well microplate (20 ul - 10 uM). Perform up to 100 PCR arrays (based on 20 ul assay volume per reaction). Just add cDNA template and SYBR green master mix. Gene List: • CCR1 chemokine (C-C motif) receptor 1 • IL11RA interleukin 11 receptor, alpha • CCR2 chemokine (C-C motif) receptor 2 • IL11RB interleukin 11 receptor, beta • CCR3 chemokine (C-C motif) receptor 3 • IL12RB1 interleukin 12 receptor, beta 1 • CCR4 chemokine (C-C motif) receptor 4 • IL12RB2 interleukin 12 receptor, beta 2 • CCR5 chemokine (C-C motif) receptor 5 • IL13RA1 interleukin 13 receptor, alpha 1 • CCR6 chemokine (C-C motif) receptor 6 • IL13RA2 interleukin 13 receptor, alpha 2 • CCR7 chemokine (C-C motif) receptor 7 • IL15RA interleukin 15 receptor, alpha • CCR8 chemokine (C-C motif) receptor 8 • IL15RB interleukin 15 receptor, beta • CCR9 chemokine (C-C motif) receptor 9 • IL17RA interleukin 17 receptor A • CCR10 chemokine (C-C motif) receptor 10 • IL17RB interleukin 17 receptor B • CX3CR1 chemokine (C-X3-C motif) receptor 1 • IL17RC interleukin 17 receptor C • CXCR1 chemokine (C-X-C motif) receptor 1 • IL17RD interleukin 17 receptor D • CXCR2 chemokine (C-X-C motif) receptor 2 • IL17RE interleukin 17 receptor E • CXCR3 chemokine (C-X-C motif) receptor 3 • IL18R1 interleukin 18 receptor