Priority Species for South Carolina State Comprehensive Wildlife
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ECOLOGY of NORTH AMERICAN FRESHWATER FISHES
ECOLOGY of NORTH AMERICAN FRESHWATER FISHES Tables STEPHEN T. ROSS University of California Press Berkeley Los Angeles London © 2013 by The Regents of the University of California ISBN 978-0-520-24945-5 uucp-ross-book-color.indbcp-ross-book-color.indb 1 44/5/13/5/13 88:34:34 AAMM uucp-ross-book-color.indbcp-ross-book-color.indb 2 44/5/13/5/13 88:34:34 AAMM TABLE 1.1 Families Composing 95% of North American Freshwater Fish Species Ranked by the Number of Native Species Number Cumulative Family of species percent Cyprinidae 297 28 Percidae 186 45 Catostomidae 71 51 Poeciliidae 69 58 Ictaluridae 46 62 Goodeidae 45 66 Atherinopsidae 39 70 Salmonidae 38 74 Cyprinodontidae 35 77 Fundulidae 34 80 Centrarchidae 31 83 Cottidae 30 86 Petromyzontidae 21 88 Cichlidae 16 89 Clupeidae 10 90 Eleotridae 10 91 Acipenseridae 8 92 Osmeridae 6 92 Elassomatidae 6 93 Gobiidae 6 93 Amblyopsidae 6 94 Pimelodidae 6 94 Gasterosteidae 5 95 source: Compiled primarily from Mayden (1992), Nelson et al. (2004), and Miller and Norris (2005). uucp-ross-book-color.indbcp-ross-book-color.indb 3 44/5/13/5/13 88:34:34 AAMM TABLE 3.1 Biogeographic Relationships of Species from a Sample of Fishes from the Ouachita River, Arkansas, at the Confl uence with the Little Missouri River (Ross, pers. observ.) Origin/ Pre- Pleistocene Taxa distribution Source Highland Stoneroller, Campostoma spadiceum 2 Mayden 1987a; Blum et al. 2008; Cashner et al. 2010 Blacktail Shiner, Cyprinella venusta 3 Mayden 1987a Steelcolor Shiner, Cyprinella whipplei 1 Mayden 1987a Redfi n Shiner, Lythrurus umbratilis 4 Mayden 1987a Bigeye Shiner, Notropis boops 1 Wiley and Mayden 1985; Mayden 1987a Bullhead Minnow, Pimephales vigilax 4 Mayden 1987a Mountain Madtom, Noturus eleutherus 2a Mayden 1985, 1987a Creole Darter, Etheostoma collettei 2a Mayden 1985 Orangebelly Darter, Etheostoma radiosum 2a Page 1983; Mayden 1985, 1987a Speckled Darter, Etheostoma stigmaeum 3 Page 1983; Simon 1997 Redspot Darter, Etheostoma artesiae 3 Mayden 1985; Piller et al. -
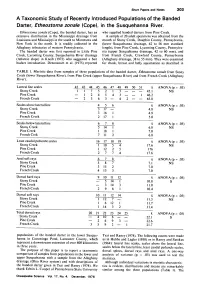
A Taxonomic Study of Recently Introduced Populations of The
Short Papers and Notes 303 A TaxonomicStudy of RecentlyIntroduced Populations of the Banded Darter,Etheostoma zonale (Cope), in the Susquehanna River. Etheostoma zonale (Cope), the banded darter, has an who supplied banded darters from Pine Creek. extensive distribution in the Mississippi drainage from A sample of 20 adult specimens was obtained from the Louisiana and Mississippi in the south to Minnesota and mouth of Stony Creek, Dauphin County, Pennsylvania, New York in the north. It is readily collected in the (lower Susquehanna drainage, 42 to 56 mm standard Allegheny tributaries of western Pennsylvania. length), from Pine Creek, Lycoming County, Pennsylva- The banded darter was first reported in Little Pine nia (upper Susquehanna drainage, 42 to 60 mm), and Creek, Lycoming County, Susquehanna River drainage from French Creek, Crawford County, Pennsylvania (Atlantic slope) in Kneib (1972) who suggested a bait (Allegheny drainage, 38 to 55 mm). They were examined bucket introduction. Denoncourt et al. (1975) reported for cheek, breast and belly squamation as described in TABLE 1. Meristic data from samples of three populations of the banded darter, Etheostoma zonale from Stony Creek (lower Susquehanna River), from Pine Creek (upper Susquehanna River) and from French Creek (Allegheny River). Lateral line scales 42 43 44 45 46 47 48 49 50 51 k ANOVA (p = .05) Stony Creek 1 1 7 5 2 1 3 - -- 45.1 NS Pine Creek - 1 2 4 5 4 3 - - 1 46.2 French Creek - 2 3 6 3 - 4 2 - - 45.8 Scales above lateral line 4 5 6 R ANOVA (p = .05) Stony Creek 3 -

Underwater Observation and Habitat Utilization of Three Rare Darters
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2010 Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee Robert Trenton Jett University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Natural Resources and Conservation Commons Recommended Citation Jett, Robert Trenton, "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee. " Master's Thesis, University of Tennessee, 2010. https://trace.tennessee.edu/utk_gradthes/636 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Robert Trenton Jett entitled "Underwater observation and habitat utilization of three rare darters (Etheostoma cinereum, Percina burtoni, and Percina williamsi) in the Little River, Blount County, Tennessee." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Wildlife and Fisheries Science. James L. Wilson, Major Professor We have read this thesis and recommend its acceptance: David A. Etnier, Jason G. -

Comparative Growth, Reproduction, Habitat and Food Utilization Of
Conservation Biology Research Grant Program Nongame Wildlife Program Division of Ecological Services Minnesota Department of Natural Resources COMPARATIVE GROWTH, REPRODUCTION, HABITAT AND FOOD UTILIZATION 0F DARTERS Of THE ST. CROIX RIVER DRAINAGE FINAL REPORT Submitted to: Lee Ann Pfannmuller Nongame Wildlife Program Minnesota Department of Natural Resources Box 7, 500 Lafayette Road St. Paul, Minnesota 55146 Submitted by: Jay T. Hatch, Ph.D. Division of Science, Business, and Mathematics General College University of Minnesota 216 Pillsbury Dr. SE Minneapolis, MN 55455 February 24, 1986 Introduction One of the most abundant and ubiquitous groups of nongame fishes found in Minnesota is the darter group (Percidae: Etheostomatini). These lively and colorful fishes inhabit nearly all of our streams and rivers and many of our lakes (Eddy and Underhill, 1974). We know in general that darters play an important role in the trophic structure of stream ecosystems (Cummins 1980), and we know that some species are important indicators of general water quality (Gerking 1945; Smith 1971; Pflieger 1975; Burr 1980; Karr 1981). Yet, we know very little about the specific life histories of the darters of our state, and we know even less about how their resource utilization patterns change with changes in habitat and community structure. To date, only three life history studies have been cared out on Minnesota darter populations. Erickson (1977) studied the banded darter (Etheostoma zonale) in the Cannon River; Coon (1982) studied several aspects of the comparative ecology of the rainbow (E. coeruleum), fantail (E. flabellare) and Johnny (E. nigrum) darters in the Root River; and Hatch (1982, 1986) studied the gilt darter (Percina evides) in the St. -

Proceedings of the United States National Museum
Proceedings of the United States National Museum SMITHSONIAN INSTITUTION . WASHINGTON, D.C. Volume 119 1966 Number 3550 CATALOG OF TYPE SPECIMENS OF THE DARTERS (PISCES, PERCIDAE, ETHEOSTOMATINI) ^ By Bruce B. Collette and Leslie W. Knapp ^ Introduction The darters are a tribe of small freshwater fishes restricted to North America. Some 212 specific and subspecific names have been pro- posed for the approximately 100 vahd described species. About a dozen species await description. As part of a long-term study of these fishes, we have prepared this catalog of type specimens. We hope that our efforts will be of value in furthering systematic research and stabilizing the nomenclature of this most fascinating group of North American fishes. In preparing this catalog we have attempted to examine or at least to verify the location of the type specimens of all nominal forms of the three presently recognized genera of darters: Percina, Ammo- crypta, and Etheostoma. By type specimens we mean holotypes, lectotypes, syntypes, paralectotypes, and paratypes. Each form is ' Fifth paper in a series on the systematics of the Percidae by the senior author. 2 Collette: Assistant Laboratory Director, Bureau of Commercial Fisheries Ichthyological Laboratory, Division of Fishes, U.S. National Museum; Knapp: Supervisor in charge of vertebrates, Oceanographic Sorting Center, Smithsonian Institution. a 2 PROCEEDINGS OF THE NATIONAL MUSEUM vol. 119 listed in alphabetical order by the generic and specific name used in the original description. If subgeneric allocation was made in the original description, that name has been placed in parentheses between the genus and the species. Holotypes or lectotypes are listed before paratypes or paralectotypes. -

Minnesota Fishes: Just How Many Species Are There Anyway?
B Spring 2015 American Currents 10 MINNESOTA FISHES: JUST HOW MANY SPECIES ARE THERE ANYWAY? Jay Hatch Dept. of Postsecondary Teaching and Learning and James Ford Bell Museum of Natural History, University of Minnesota INTRODUCTION FIGURING OUT THE COUNT In terms of fish diversity, for a state at the northern edge and Were they ever really here? halfway between the east–west extremes of the contiguous On the surface, this one appears pretty simple, but it can USA, Minnesota doesn’t do badly. Of the five states and two cause way more gray hairs than you might think. For ex- Canadian provinces bordering it, only Wisconsin boasts as ample, what do you do if Minnesota’s ichthyological fore- many or more species. We (my fish biology colleagues and )I fathers—like Albert Woolman and Ulysses Cox—reported believe this is true, but counting species is not quite as easy species such as the Chestnut Lamprey (Icthyomyzon casta- as it seems. You’re asking: What could be easier? Just find out neus) from the Minnesota River basin or the Longnose Gar if a fish species swims in your lakes or streams, then count it, (Lepisosteus osseus) from the Red River of the North basin right? Well, as they used to say in the Hertz rental car com- (see Figure 1 for Minnesota’s 10 major basins), but no one mercial, “not exactly.” else has ever collected these species in those basins over the What kinds of issues lead to “not exactly?” Quite a few, last 120 years? Look at the specimens, right? Good luck; including the uncertainty of old or historical records, the they no longer exist. -

Geological Survey of Alabama Calibration of The
GEOLOGICAL SURVEY OF ALABAMA Berry H. (Nick) Tew, Jr. State Geologist ECOSYSTEMS INVESTIGATIONS PROGRAM CALIBRATION OF THE INDEX OF BIOTIC INTEGRITY FOR THE SOUTHERN PLAINS ICHTHYOREGION IN ALABAMA OPEN-FILE REPORT 1210 by Patrick E. O'Neil and Thomas E. Shepard Prepared in cooperation with the Alabama Department of Environmental Management and the Alabama Department of Conservation and Natural Resources Tuscaloosa, Alabama 2012 TABLE OF CONTENTS Abstract ............................................................ 1 Introduction.......................................................... 2 Acknowledgments .................................................... 6 Objectives........................................................... 7 Study area .......................................................... 7 Southern Plains ichthyoregion ...................................... 7 Methods ............................................................ 9 IBI sample collection ............................................. 9 Habitat measures............................................... 11 Habitat metrics ........................................... 12 The human disturbance gradient ................................... 16 IBI metrics and scoring criteria..................................... 20 Designation of guilds....................................... 21 Results and discussion................................................ 23 Sampling sites and collection results . 23 Selection and scoring of Southern Plains IBI metrics . 48 Metrics selected for the -

ON 23(3) 461-466.Pdf
SHORT COMMUNICATIONS ORNITOLOGIA NEOTROPICAL 23: 461–466, 2012 © The Neotropical Ornithological Society FIRST DESCRIPTION OF THE NEST AND EGGS OF THE PLAIN-FLANKED RAIL (RALLUS WETMOREI) Adriana Rodríguez-Ferraro1,2, Eugenia Sánchez2, & Miguel Lentino3 1Departamento de Estudios Ambientales, Universidad Simón Bolívar, Apdo. 89.000, Caracas 1080-A, Venezuela. E-mail: [email protected] 2Laboratorio de Ecología Molecular de Vertebrados, Universidad Simón Bolívar, Apdo. 89.000, Caracas 1080-A, Venezuela. 3Fundación William H. Phelps, Apdo. 2009, Caracas 1010-A, Venezuela. Primera descripción del nido y los huevos de la Polla de Wetmore (Rallus wetmorei). Key words: Plain-flanked Rail, Rallus wetmorei, eggs, mangrove, nest, Venezuela. INTRODUCTION conservation priorities in Venezuela (Rodrí- guez et al. 2004). Main threats for this rail The Plain-flanked Rail (Rallus wetmorei) is a are the loss and deterioration of mangrove Venezuelan endemic species deserving urgent habitat as a consequence of expanding attention from a conservation perspective. touristic developments and activities derived This bird was first described in the mid-1940s from petrochemical industries (Rodríguez & (Zimmer & Phelps 1944), and after a few Rojas-Suárez 2008). These problems even other observations during the following 10 exist within the boundaries of the few pro- years, it went unrecorded for almost three tected areas (three national parks and one decades, until rediscovered in 1999 (Hilty wildlife refuge) where the species is known to 2003). It is restricted to a small area along the occur. central coast of Venezuela where it is known Recovery efforts of endangered birds from eight localities (Taylor 1996), but in have been hampered by the lack of basic recent years, it has been found in only five of knowledge on their biology, thus, research these sites (Rodríguez-Ferraro & Lentino in focused on determining biological character- prep.). -

Recovery Strategy for the King Rail (Rallus Elegans) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Recovery Strategy for the King Rail (Rallus elegans) in Canada King Rail 2010 Recommended citation: Environment Canada. 2010. Recovery Strategy for the King Rail (Rallus elegans) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. vi + 21 pp. For copies of the recovery strategy, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk Public Registry (www.sararegistry.gc.ca). Cover illustration: U.S. Fish & Wildlife Service Également disponible en français sous le titre « Programme de rétablissement du Râle élégant (Rallus elegans) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of Environment, 2010. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. Recovery Strategy for the King Rail 2010 PREFACE The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of recovery strategies for listed Extirpated, Endangered, and Threatened species and are required to report on progress within five years. The Minister of the Environment and the Minister responsible for the Parks Canada Agency are the competent ministers for the recovery of the King Rail and have prepared this strategy, as per section 37 of SARA. -

Fish Species List
FISH American brook lamprey Lampetra appendix American eel Anguilla rostrata Banded darter Etheostoma zonale Bigeye chub Notropis amblops Bigeye shiner Notropis boops Bigmouth buffalo Ictiobus cyprinellus Bigmouth shiner Notropis dorsalis Black buffalo Ictiobus niger Black bullhead Ameiurus melas Black crappie Pomoxis nigromaculatus Black redhorse Moxostoma duquesnei Blacknose dace Rhinichthys atratulus Blackside darter Percina maculate Blackstripe topminnow Fundulus notatus Blue sucker Cycleptus elongates Bluebreast darter Etheostoma camurum Bluegill Lepomis macrochirus Bluntnose minnow Pimephales notatus Bowfin Amia calva Brindled madtom Noturus miurus Brook silverside Labidesthes sicculus Brook stickleback Culaea inconstans Brook trout Salvelinus fontinalis Brown bullhead Ictalurus nebulosus Bullhead minnow Pimephales vigilax Central mudminnow Umbra limi Central stoneroller Campostoma anomalum Channel catfish Ictalurus punctatus Channel darter Percina copelandi Channel shiner Notropis wickliffi Common shiner Luxilus cornutus Creek chub Semotilus atromaculatus Creek chubsucker Erimyzon oblongus Dusky darter Percina sciera sciera Eastern sand darter Ammocrypta pellucida Emerald shiner Notropis atherinoides Fantail darter Etheostoma flabellare Fathead minnow Pimephales promelas Flathead catfish Pylodictis olivaris Freshwater drum Aplodinotus grunniens Ghost shiner Notropis buchanani Gizzard shad Dorosoma cepedianum Golden redhorse Moxostoma erythrurum Golden shiner Notemigonus crysoleucas Goldeye Hiodon alosoides Grass pickerel Esox americanus -
![Kyfishid[1].Pdf](https://docslib.b-cdn.net/cover/2624/kyfishid-1-pdf-1462624.webp)
Kyfishid[1].Pdf
Kentucky Fishes Kentucky Department of Fish and Wildlife Resources Kentucky Fish & Wildlife’s Mission To conserve, protect and enhance Kentucky’s fish and wildlife resources and provide outstanding opportunities for hunting, fishing, trapping, boating, shooting sports, wildlife viewing, and related activities. Federal Aid Project funded by your purchase of fishing equipment and motor boat fuels Kentucky Department of Fish & Wildlife Resources #1 Sportsman’s Lane, Frankfort, KY 40601 1-800-858-1549 • fw.ky.gov Kentucky Fish & Wildlife’s Mission Kentucky Fishes by Matthew R. Thomas Fisheries Program Coordinator 2011 (Third edition, 2021) Kentucky Department of Fish & Wildlife Resources Division of Fisheries Cover paintings by Rick Hill • Publication design by Adrienne Yancy Preface entucky is home to a total of 245 native fish species with an additional 24 that have been introduced either intentionally (i.e., for sport) or accidentally. Within Kthe United States, Kentucky’s native freshwater fish diversity is exceeded only by Alabama and Tennessee. This high diversity of native fishes corresponds to an abun- dance of water bodies and wide variety of aquatic habitats across the state – from swift upland streams to large sluggish rivers, oxbow lakes, and wetlands. Approximately 25 species are most frequently caught by anglers either for sport or food. Many of these species occur in streams and rivers statewide, while several are routinely stocked in public and private water bodies across the state, especially ponds and reservoirs. The largest proportion of Kentucky’s fish fauna (80%) includes darters, minnows, suckers, madtoms, smaller sunfishes, and other groups (e.g., lam- preys) that are rarely seen by most people. -

Edna) Assay Leads to New Insights on Ruffe (Gymnocephalus Cernua) Spread in North America
Biol Invasions (2016) 18:3205–3222 DOI 10.1007/s10530-016-1209-z ORIGINAL PAPER A sensitive environmental DNA (eDNA) assay leads to new insights on Ruffe (Gymnocephalus cernua) spread in North America Andrew J. Tucker . W. Lindsay Chadderton . Christopher L. Jerde . Mark A. Renshaw . Karen Uy . Crysta Gantz . Andrew R. Mahon . Anjanette Bowen . Timothy Strakosh . Jonathan M. Bossenbroek . Jennifer L. Sieracki . Dmitry Beletsky . Jennifer Bergner . David M. Lodge Received: 7 July 2015 / Accepted: 27 June 2016 / Published online: 6 July 2016 Ó The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Detection of invasive species before or the Great Lakes, we designed an eDNA surveillance soon after they establish in novel environments is study to target Ruffe at the putative leading edge of the critical to prevent widespread ecological and eco- invasion. We report a much more advanced invasion nomic impacts. Environmental DNA (eDNA) surveil- front for Ruffe than has been indicated by conven- lance and monitoring is an approach to improve early tional surveillance methods and we quantify rates of detection efforts. Here we describe a large-scale false negative detections (i.e. failure to detect DNA conservation application of a quantitative polymerase when it is present in a sample). Our results highlight chain reaction assay with a case study for surveillance the important role of eDNA surveillance as a sensitive of a federally listed nuisance species (Ruffe, Gymno- tool to improve early detection efforts for aquatic cephalus cernua) in the Laurentian Great Lakes. Using invasive species and draw attention to the need for an current Ruffe distribution data and predictions of improved understanding of detection errors.