Caresource Pharmacy Policy Statement Marketplace Amevive (Alefacept)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -

Prescription Drugs Requiring Prior Authorization
PRESCRIPTION DRUGS REQUIRING PRIOR AUTHORIZATION Revised 10/16 As part of our drug utilization management program, members must request and receive prior authorization for certain prescription drugs in order to use their prescription drug benefits. Below is a list of drugs that currently require prior authorization. This list will be updated periodically as new drugs that require prior authorization are introduced. As benefits may vary by group and individual plans, the inclusion of a medication on this list does not imply prescription drug coverage. The Schedule of Benefits contains a list of drug categories that require prior authorization. Prior authorization requests are processed by our pharmacy benefit manager, Express Scripts, Inc. (ESI). Physicians must call ESI to obtain an authorization. (1-800-842-2015). Drug Name Generic Name Drug Classification Abstral fentanyl citrate oral tablet Controlled Dangerous Substance Accu-Chek Test Strips blood glucose test strips Blood Glucose Test Strips Actemra tocilizumab Monoclonal Antibody Acthar corticotropin Hormone Actimmune interferon gamma 1b Interferon Actiq fentanyl citrate OTFC Controlled Dangerous Substance Adcirca tadalafil Pulmonary Vasodilator Adempas riociguat Pulmonary Vasodilator Adlyxin lixisenatide Type 2 Diabetes Advocate Test Strips blood glucose test strips Blood Glucose Test Strips Aerospan** flunisolide Corticosteroids (Inhaled) Afrezza insulin Insulin (inhaled) Ampyra dalfampridine Multiple Sclerosis Agent Altoprev** lovastatin Cholesterol Alvesco** ciclesonide Corticosteroids -

The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy
Review Neuromuscular Diseases Early Diagnosis and Treatment – The Use of Ataluren in the Effective Management of Duchenne Muscular Dystrophy Eugenio Mercuri,1 Ros Quinlivan2 and Sylvie Tuffery-Giraud3 1. Catholic University, Rome, Italy; 2. Great Ormond Street Hospital and National Hospital for Neurology and Neurosurgery, London, UK; 3. Laboratory of Genetics of Rare Diseases (LGMR), University of Montpellier, Montpellier, France DOI: https://doi.org/10.17925/ENR.2018.13.1.31 he understanding of the natural history of Duchenne muscular dystrophy (DMD) is increasing rapidly and new treatments are emerging that have the potential to substantially improve the prognosis for patients with this disabling and life-shortening disease. For many, Thowever, there is a long delay between the appearance of symptoms and DMD diagnosis, which reduces the possibility of successful treatment. DMD results from mutations in the large dystrophin gene of which one-third are de novo mutations and two-thirds are inherited from a female carrier. Roughly 75% of mutations are large rearrangements and 25% are point mutations. Certain deletions and nonsense mutations can be treated whereas many other mutations cannot currently be treated. This emphasises the need for early genetic testing to identify the mutation, guide treatment and inform genetic counselling. Treatments for DMD include corticosteroids and more recently, ataluren has been approved in Europe, the first disease-modifying therapy for treating DMD caused by nonsense mutations. The use of ataluren in DMD is supported by positive results from phase IIb and phase III studies in which the treatment produced marked improvements in the 6-minute walk test, timed function tests such as the 10 m walk/run test and the 4-stair ascent/descent test compared with placebo. -

Animal Models of Duchenne Muscular Dystrophy: from Basic Mechanisms to Gene Therapy Joe W
© 2015. Published by The Company of Biologists Ltd | Disease Models & Mechanisms (2015) 8, 195-213 doi:10.1242/dmm.018424 REVIEW Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy Joe W. McGreevy1, Chady H. Hakim1, Mark A. McIntosh1 and Dongsheng Duan1,2,* ABSTRACT The identification of the disease-causing gene and the molecular Duchenne muscular dystrophy (DMD) is a progressive muscle- basis for the DMD and BMD phenotypes establishes the foundation wasting disorder. It is caused by loss-of-function mutations in the for DMD gene therapy (Fig. 2A). To mitigate muscle disease, one dystrophin gene. Currently, there is no cure. A highly promising can either restore the full-length transcript or express a truncated but therapeutic strategy is to replace or repair the defective dystrophin in-frame dystrophin gene (Duan, 2011; Goyenvalle et al., 2011; gene by gene therapy. Numerous animal models of DMD have been Konieczny et al., 2013; Mendell et al., 2012; Verhaart and Aartsma- developed over the last 30 years, ranging from invertebrate to large Rus, 2012). Several gene therapy strategies are currently under mammalian models. mdx mice are the most commonly employed development. They include replacing the mutated gene with a models in DMD research and have been used to lay the groundwork functional candidate gene (gene replacement) or repairing the for DMD gene therapy. After ~30 years of development, the field has defective gene by targeted correction and exon skipping (gene reached the stage at which the results in mdx mice can be validated repair). Currently, adeno-associated virus (AAV)-mediated gene and scaled-up in symptomatic large animals. -
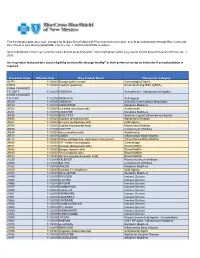
Procedure Code Effective Date Drug Product Name* Therapeutic
This list includes procedure code changes for Medical Benefit Specialty Pharmacy that may require benefit preauthorization through Blue Cross and Blue Shield of New Mexico (BCBSNM) effective Jan. 1, 2020 for BCBSNM members. Items highlighted in blue may currently require benefit preauthorization. Items highlighted yellow may require benefit preauthorization effective Jan. 1, 2020. It is imperative that providers check eligibility and benefits through Availity® or their preferred vendor to determine if preauthorization is required. Procedure Code Effective Date Drug Product Name* Therapeutic Category 90378 1/1/2020 Synagis (palivizumab) Immunological Agent C9036 1/1/2020 Onpattro (patisiran) Small interfering RNA (siRNA) C9466 CHANGED TO J0517 1/1/2019 FASENRA Antiasthmatic - Monoclonal Antibodies C9493 CHANGED TO J1301 1/1/2019 RADICAVA ALS Agents J0129 1/1/2019 ORENCIA Selective Costimulation Modulators J0180 1/1/2019 FABRAZYME Metabolic Modifiers J0202 1/1/2020 Lemtrada (alemtuzumab) Autoimmune J0221 1/1/2019 LUMIZYME Metabolic Modifiers J0490 1/1/2019 BENLYSTA Systemic Lupus Erythematosus Agents J0565 1/1/2020 Zinplava (bezlotoxumab) Monoclonal Antibody J0567 1/1/2020 Brineura (cerliponase alfa) CNS Agents J0584 1/1/2020 Crysvita (burosumab-twza) Monoclonal Antibody J0598 1/1/2019 CINRYZE Complement Inhibitors J0638 1/1/2020 Ilaris (canakinumab) Autoimmune J0717 1/1/2019 CIMZIA Inflammatory Bowel Agents J0775 1/1/2020 Xiaflex (collagenase, clostridium histolyticum) Tissue Permeability Modifier J0800 1/1/2020 H.P. Acthar (corticotropin) -

Med Pharm PA List to Build Pdfs.Xlsx
The following medications, typically administered in a provider's office or ambulatory/outpatient infusion center, require authorization prior to utilization. Authorization requests can be submitted via the Portal. Additionally, you are welcome to call our member services line to initiate the Prior Authorization process. This list is updated quaterly. Effective date: 1/1/2021 Therapeutic category Brand Name Generic Name HCPCS Immunologic agent Orencia, IV infusion abatacept J0129 Botulinum toxins Dysport abobotulinumtoxin B J0586 HER-2 receptor drug Kadcyla ado-trastuzumab emtansine J9354 Ophthalmic injectable Eylea afilbercept J0178 Enzyme replacement drug Fabrazyme agalsidase beta J0180 Multiple sclerosis drug Lemtrada alemtuzumab J0202 Enzyme replacement drug Lumizyme alglucosidase alfa J0221 Enzyme replacement drug Strensiq asfotase alfa J3590 (non-specific) PD1-PDL1 drug Tecentriq atezolizumab J9022 PD1-PDL1 drug Bavencio avelumab J9023 CAR-T Therapy Yescarta axicabtageneciloleucel inpatient admin Systemic lupus erythematosus (SLE) drug Benlysta belimumab J0490 Antineoplastic agent Beleodaq belinostat J9032 Antineoplastic Belrapzo bendamustine HCl J9036 Antineoplastic Bendeka bendamustine HCl J9034 Antineoplastic agent Treanda bendamustine HCl J9033 Respiratory injectable Fasenra benralizumab J0517 J9035/J3590 (ophthalmology Antineoplastic Avastin bevacizumab uses this non-specific code) Antineoplastic agent Mvasi bevacizumab-awwb Q5107 Antineoplastic agent Zirabev bevacizumab-bvzr Q5118 Antineoplastic agent Blincyto blinatumomab -

Deflazacort, Eteplirsen, and Golodirsen for Duchenne Muscular Dystrophy: Effectiveness and Value
Deflazacort, Eteplirsen, and Golodirsen for Duchenne Muscular Dystrophy: Effectiveness and Value Final Evidence Report August 15, 2019 Prepared for ©Institute for Clinical and Economic Review, 2019 University of Illinois at Chicago College of Pharmacy ICER Staff and Consultants Modeling Group Grace A. Lin, MD Catherine Koola, MPH Surrey M. Walton, PhD Associate Professor of Program Manager Associate Professor, Pharmacy Systems, Outcomes and Policy Medicine and Health Policy ICER Assistant Director, Center for Pharmacoepidemiology and University of California, San Pharmacoeconomic Research Francisco University of Illinois at Chicago College of Pharmacy Foluso Agboola, MBBS, MPH Matt Seidner Nicole Boyer, PhD Director, Evidence Synthesis Program Director Postdoctoral Fellow ICER ICER The University of Chicago Noemi Fluetsch, MPH Rick Chapman, PhD, MS Danny Quach, PharmD Research Assistant, Health Director of Health Economics University of Illinois at Chicago College of Pharmacy Economics and Outcomes ICER Research ICER Varun M. Kumar, MBBS, MPH, David Rind, MD, MSc MSc Chief Medical Officer Associate Director of Health ICER Economics ICER The role of the University of Illinois (UIC) College of Pharmacy Sumeyye Samur, PhD, MS Steve Pearson, MD, MSc Modeling Group is limited to the development of the cost- Health Economist President effectiveness model, and the resulting ICER reports do not ICER ICER necessarily represent the views of the UIC. DATE OF PUBLICATION: August 15, 2019 Grace Lin served as the lead author for the report and wrote the background, other benefits, and contextual considerations sections of the report. Foluso Agboola was responsible for the oversight of the systematic review and authorship of the comparative clinical effectiveness section with the support of Ifeoma Otuonye and Noemi Fluetsch. -

What Is the Level of Dystrophin Expression Required for Effective
RVC OPEN ACCESS REPOSITORY – COPYRIGHT NOTICE This is the author’s accepted manuscript. The version of record is available online via the Journal of Muscle Research and Cell Motility: https://doi.org/10.1007/s10974-019-09535-9. The full details of the published version of the article are as follows: TITLE: What is the level of dystrophin expression required for effective therapy of Duchenne muscular dystrophy? AUTHORS: DJ Wells JOURNAL TITLE: Journal of Muscle Research and Cell Motility PUBLICATION DATE: 9 July 2019 PUBLISHER: Springer Nature DOI: 10.1007/s10974-019-09535-9 What is the level of dystrophin expression required for effective therapy of Duchenne muscular dystrophy? Dominic J. Wells. Neuromuscular Diseases Group, Department of Comparative Biomedical Sciences, Royal Veterinary College, Royal College Street NW1 0TU, UK. [email protected] orcid.org/0000-0002-1425-6344 Abstract Duchenne muscular dystrophy (DMD) is a fatal X-linked muscle wasting disease. The disease is due to mutations in the DMD gene that encodes for a large intracellular protein called dystrophin. Dystrophin plays a critical role in linking the internal cytoskeleton of the striated muscle cell with the extracellular matrix as well as having cell signalling functions. In its absence muscle contraction is associated with cycles of damage, repair, inflammation and fibrosis with eventual loss of muscle and replacement with fat. Experiments in animal models of DMD have generated a number of different approaches to the induction of dystrophin including viral vector mediated delivery of a recombinant dystrophin gene, antisense oligonucleotide mediated exon-skipping to restore the open reading frame in the dystrophin mRNA, read-through of premature stop mutations, genome modification using CRISPR-Cas9 or cell based transfer of a functional dystrophin gene. -

ENTRY WATCH 2016 Published by the Patented Medicine Prices Review Board June 2018 Meds Entry Watch, 2016 Is Available in Electronic Format on the PMPRB Website
MEDS ENTRY WATCH 2016 Published by the Patented Medicine Prices Review Board June 2018 Meds Entry Watch, 2016 is available in electronic format on the PMPRB website. Une traduction de ce document est également disponible en français sous le titre : Veille des médicaments mis en marché, 2016 Patented Medicine Prices Review Board Standard Life Centre Box L40 333 Laurier Avenue West Suite 1400 Ottawa, ON K1P 1C1 Tel.: 1-877-861-2350 TTY 613-288-9654 Email: [email protected] Web: www.pmprb-cepmb.gc.ca ISSN 2560-6204 Cat. No.: H79-12E-PDF © Her Majesty the Queen in Right of Canada, as represented by the NPDUIS initiative of the Patented Medicine Prices Review Board, 2018 MEDS ENTRY WATCH 2016 About the PMPRB Acknowledgements The Patented Medicine Prices Review Board This report was prepared by the Patented (PMPRB) is a respected public agency that makes Medicine Prices Review Board (PMPRB) a unique and valued contribution to sustainable as part of the National Prescription Drug spending on pharmaceuticals in Canada by: Utilization Information System (NPDUIS). ~ providing stakeholders with price, cost and The PMPRB would like to acknowledge the utilization information to help them make timely contributions of and knowledgeable drug pricing, purchasing and ~ The members of the NPDUIS Advisory reimbursement decisions; and Committee for their expert oversight and ~ acting as an effective check on the patent rights guidance in the preparation of this report. of pharmaceutical manufacturers through the ~ PMPRB NPDUIS staff for their contribution responsible and efficient use of its consumer to the analytical content of the report: protection powers. -

Aetna PA Info
Procedures, programs and drugs that require precertification Participating provider precertification list Starting June 1, 2021 Applies to the following plans (also see General information section #1-#4, #9-#10): Aetna® plans, except Traditional Choice® plans All health benefits and insurance plans offered and/or underwritten by Innovation Health plans, Inc., and Innovation Health Insurance Company, except indemnity plans, Foreign Service Benefit Plan, MHBP and Rural Carrier Benefit Plan All health benefits and health insurance plans offered, underwritten and/or administered by the following: Banner Health and Aetna Health Insurance Company and/or Banner Health and Aetna Health Plan Inc. (Banner|Aetna), Texas Health +Aetna Health Insurance Company and/or Texas Health+Aetna Health Plan Inc. (Texas Health Aetna), Allina Health and Aetna Health Insurance Company (Allina Health| Aetna), Sutter Health and Aetna Administrative Services LLC (Sutter Health | Aetna) Aetna.com 23.03.882.1 R (6/21) For more information, read all general precertification guidelines Providers may submit most precertification requests electronically through the secure provider website or using your Electronic Medical Record (EMR) system portal. (See #1 in the General Information section for more information on precertification.) Services that require precertification: 1. Inpatient confinements (except hospice) 18. Nonparticipating freestanding ambulatory For example, surgical and nonsurgical stays, surgical facility services, when referred by stays in a skilled nursing facility or rehabilitation a participating provider facility, and maternity and newborn stays that 19. Orthognathic surgery procedures, bone exceed the standard length of stay (LOS). (See grafts, osteotomies and surgical #6 in the General Information section.) management of the temporomandibular 2. Ambulance joint Precertification required for transportation by 20. -

News & Analysis
NEWS & ANALYSIS NEWS IN BRIEF Industry’s interest in RNA- targeted FDA approves first BCMA- targeted therapeutic small- molecule drugs is growing. In April, The FDA has approved GlaxoSmithKline on the drug’s label notes the associated risk Roche partnered with Arrakis Therapeutics (GSK)’s belantamab mafodotin for of severe vision loss. on RNA- targeted small-molecule drug relapsed or refractory multiple myeloma. GSK is still developing the ADC for earlier discovery. Other biotechs that are working The antibody–drug conjugate (ADC) is lines of therapy, and in combination with preclinically on RNA-targeted small molecules the first therapeutic from the crowded other agents. A benefit of the ADC approach include Expansion Therapeutics, Skyhawk BCMA- targeted pipeline to secure approval. is that it provides an off-the- shelf product. Therapeutics and Ribometrix. BCMA, a member of the TNF-receptor BCMA- targeted CAR- T therapies that Asher Mullard superfamily, is expressed on normal B are made to order for each patient are also lymphocytes as well as on multiple myeloma approaching the market, setting the stage FDA approves first GPCR cells. Despite early efforts to target BCMA for a modality showdown. Bristol Myers with canonical monoclonal antibodies, early Squibb and Bluebird Bio resubmitted their biased agonist candidates did not have sufficient efficacy idecabtagene vicleucel for FDA approval The FDA has approved Trevena’s μ- opioid to move forward. In recent years, however, in July, following an earlier submission agonist oliceridine for moderate to severe drug developers have had more success with and refuse- to- file letter. Johnson & Johnson acute pain in adults. -

Der Einfluss Der VO (EG) 1901/2006 Auf Den Off-Label Use in Der Neonatologie“
DIPLOMARBEIT / DIPLOMA THESIS Titel der Diplomarbeit / Title of the Diploma Thesis „Der Einfluss der VO (EG) 1901/2006 auf den Off-Label Use in der Neonatologie“ verfasst von / submitted by Stefanie Bub angestrebter akademischer Grad / in partial fulfilment of the requirements for the degree of Magistra der Pharmazie (Mag.pharm.) Wien, 2016 / Vienna, 2016 Studienkennzahl lt. Studienblatt / A 449 degree programme code as it appears on the student record sheet: Studienrichtung lt. Studienblatt / Diplomstudium Pharmazie degree programme as it appears on the student record sheet: Betreut von / Supervisor: Ao. Univ.-Prof. Mag. Dr. Rosa Lemmens Danksagung Als erstes möchte ich mich bei Frau Prof. Dr. Rosa Lemmens für die Betreuung meiner Diplomarbeit bedanken. Durch sie war es mir erst möglich, dieses Thema zu behandeln und meine Interessen zu verwirklichen. Ganz besonders möchte ich auch Dr. Andreas Doschek für seine jahrelange Unterstützung in diesem Studium danken. Abgesehen von den abertausenden Kopien und Ausdrucken, die er mir und meinen MitstudentInnen zur Verfügung gestellt hat, hat er mir vor jeder Prüfung den Rücken freigehalten, mich abgeprüft und mir so das Studium sehr erleichtert. Ihm habe ich auch das Interesse für die Materie des Arzneimittelrechts zu verdanken, weswegen ich mich für dieses Thema in meiner Abschlußarbeit entschieden habe. Meiner Familie und meinen Freunden danke ich für die Motivation und die guten Ratschläge und meinen MitstudentInnen für eine wundervolle Zeit. Danke! Wien, September 2016 I II Inhaltsverzeichnis Seite 1. Einführung 1 2. Grundlagen der zulassungsüberschreitenden Arzneimittelanwendung 4 2.1. Begriffsdefinitionen 4 2.1.1. Random controlled trial (RCT) 4 2.1.2. Pharmakokinetik-Studien 4 2.1.3.